This is a preprint.
Smad4 is essential for epiblast scaling and morphogenesis after implantation, but nonessential prior to implantation in the mouse
- PMID: 38328075
- PMCID: PMC10849569
- DOI: 10.1101/2024.01.23.576717
Smad4 is essential for epiblast scaling and morphogenesis after implantation, but nonessential prior to implantation in the mouse
Update in
-
Smad4 is essential for epiblast scaling and morphogenesis after implantation, but nonessential before implantation.Development. 2024 Jun 1;151(11):dev202377. doi: 10.1242/dev.202377. Epub 2024 Jun 5. Development. 2024. PMID: 38752427 Free PMC article.
Abstract
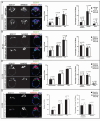
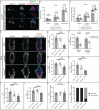
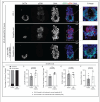
Bone Morphogenic Protein (BMP) signaling plays an essential and highly conserved role in axial patterning in embryos of many externally developing animal species. However, in mammalian embryos, which develop inside the mother, early development includes an additional stage known as preimplantation. During preimplantation, the epiblast lineage is segregated from the extraembryonic lineages that enable implantation and development in utero. Yet, the requirement for BMP signaling in mouse preimplantation is imprecisely defined. We show that, in contrast to prior reports, BMP signaling (as reported by SMAD1/5/9 phosphorylation) is not detectable until implantation, when it is detected in the primitive endoderm - an extraembryonic lineage. Moreover, preimplantation development appears normal following deletion of maternal and zygotic Smad4, an essential effector of BMP signaling. In fact, mice lacking maternal Smad4 are viable. Finally, we uncover a new requirement for zygotic Smad4 in epiblast scaling and cavitation immediately after implantation, via a mechanism involving FGFR/ERK attenuation. Altogether, our results demonstrate no role for BMP4/SMAD4 in the first lineage decisions during mouse development. Rather, multi-pathway signaling among embryonic and extraembryonic cell types drives epiblast morphogenesis post-implantation.
Keywords: epiblast; extraembryonic; maternal and zygotic gene deletion; morphogenesis; pSMAD1/5/9.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures


References
-
- Beppu H., Kawabata M., Hamamoto T., Chytil A., Minowa O., Noda T. and Miyazono K. (2000). BMP type II receptor is required for gastrulation and early development of mouse embryos. Developmental Biology 221, 249–258. - PubMed
-
- Brennan J., Lu C.C., Norris D. P., Rodriguez T.A., Beddington R.S.P. and Robertson E.J. (2001). Nodal signalling in the epiblast patterns the early mouse embryo. Nature 411, 965–969. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
