This is a preprint.
The structural and mechanistic bases for the viral resistance to allosteric HIV-1 integrase inhibitor pirmitegravir
- PMID: 38328097
- PMCID: PMC10849636
- DOI: 10.1101/2024.01.26.577387
The structural and mechanistic bases for the viral resistance to allosteric HIV-1 integrase inhibitor pirmitegravir
Update in
-
The structural and mechanistic bases for the viral resistance to allosteric HIV-1 integrase inhibitor pirmitegravir.mBio. 2024 Nov 13;15(11):e0046524. doi: 10.1128/mbio.00465-24. Epub 2024 Oct 15. mBio. 2024. PMID: 39404354 Free PMC article.
Abstract
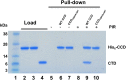
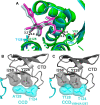
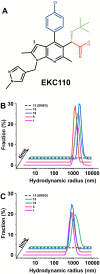
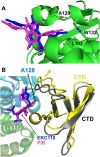
Allosteric HIV-1 integrase (IN) inhibitors (ALLINIs) are investigational antiretroviral agents which potently impair virion maturation by inducing hyper-multimerization of IN and inhibiting its interaction with viral genomic RNA. The pyrrolopyridine-based ALLINI pirmitegravir (PIR) has recently advanced into Phase 2a clinical trials. Previous cell culture based viral breakthrough assays identified the HIV-1(Y99H/A128T IN) variant that confers substantial resistance to this inhibitor. Here, we have elucidated the unexpected mechanism of viral resistance to PIR. While both Tyr99 and Ala128 are positioned within the inhibitor binding V-shaped cavity at the IN catalytic core domain (CCD) dimer interface, the Y99H/A128T IN mutations did not substantially affect direct binding of PIR to the CCD dimer or functional oligomerization of full-length IN. Instead, the drug-resistant mutations introduced a steric hindrance at the inhibitor mediated interface between CCD and C-terminal domain (CTD) and compromised CTD binding to the CCDY99H/A128T + PIR complex. Consequently, full-length INY99H/A128T was substantially less susceptible to the PIR induced hyper-multimerization than the WT protein, and HIV-1(Y99H/A128T IN) conferred >150-fold resistance to the inhibitor compared to the WT virus. By rationally modifying PIR we have developed its analog EKC110, which readily induced hyper-multimerization of INY99H/A128T in vitro and was ~14-fold more potent against HIV-1(Y99H/A128T IN) than the parent inhibitor. These findings suggest a path for developing improved PIR chemotypes with a higher barrier to resistance for their potential clinical use.
Keywords: ALLINI; HIV-1 integrase; Pirmitegravir; antiretroviral drug.
Conflict of interest statement
Conflict of interest statement The authors declare a conflict of interest. Kyungjin Kim is the Chief Executive Officer of ST Pharm Co. Ltd. No other authors declare a potential conflict of interest.
Figures




References
-
- Christ F, Voet A, Marchand A, Nicolet S, Desimmie BA, Marchand D, Bardiot D, Van der Veken NJ, Van Remoortel B, Strelkov SV, De Maeyer M, Chaltin P, Debyser Z. 2010. Rational design of small-molecule inhibitors of the LEDGF/p75-integrase interaction and HIV replication. Nat Chem Biol 6:442–8. - PubMed
-
- Fader LD, Malenfant E, Parisien M, Carson R, Bilodeau F, Landry S, Pesant M, Brochu C, Morin S, Chabot C, Halmos T, Bousquet Y, Bailey MD, Kawai SH, Coulombe R, LaPlante S, Jakalian A, Bhardwaj PK, Wernic D, Schroeder P, Amad M, Edwards P, Garneau M, Duan J, Cordingley M, Bethell R, Mason SW, Bos M, Bonneau P, Poupart MA, Faucher AM, Simoneau B, Fenwick C, Yoakim C, Tsantrizos Y. 2014. Discovery of BI 224436, a Noncatalytic Site Integrase Inhibitor (NCINI) of HIV-1. ACS Med Chem Lett 5:422–7. - PMC - PubMed
-
- Patel D, Antwi J, Koneru PC, Serrao E, Forli S, Kessl JJ, Feng L, Deng N, Levy RM, Fuchs JR, Olson AJ, Engelman AN, Bauman JD, Kvaratskhelia M, Arnold E. 2016. A New Class of Allosteric HIV-1 Integrase Inhibitors Identified by Crystallographic Fragment Screening of the Catalytic Core Domain. J Biol Chem 291:23569–23577. - PMC - PubMed
-
- Maehigashi T, Ahn S, Kim UI, Lindenberger J, Oo A, Koneru PC, Mahboubi B, Engelman AN, Kvaratskhelia M, Kim K, Kim B. 2021. A highly potent and safe pyrrolopyridine-based allosteric HIV-1 integrase inhibitor targeting host LEDGF/p75-integrase interaction site. PLoS Pathog 17:e1009671. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
