This is a preprint.
Burkholderia cenocepacia epigenetic regulator M.BceJIV simultaneously engages two DNA recognition sequences for methylation
- PMID: 38328099
- PMCID: PMC10849533
- DOI: 10.1101/2024.01.20.576384
Burkholderia cenocepacia epigenetic regulator M.BceJIV simultaneously engages two DNA recognition sequences for methylation
Update in
-
Burkholderia cenocepacia epigenetic regulator M.BceJIV simultaneously engages two DNA recognition sequences for methylation.Nat Commun. 2024 Sep 7;15(1):7839. doi: 10.1038/s41467-024-52130-x. Nat Commun. 2024. PMID: 39244607 Free PMC article.
Abstract
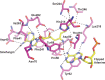
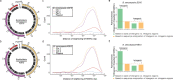
Burkholderia cenocepacia is an opportunistic and infective bacterium containing an orphan DNA methyltransferase (M.BceJIV) with roles in regulating gene expression and motility of the bacterium. M.BceJIV recognizes a GTWWAC motif (where W can be an adenine or a thymine) and methylates the N6 of the adenine at the fifth base position (GTWWAC). Here, we present a high-resolution crystal structure of M.BceJIV/DNA/sinefungin ternary complex and allied biochemical, computational, and thermodynamic analyses. Remarkably, the structure shows not one, but two DNA substrates bound to the M.BceJIV dimer, wherein each monomer contributes to the recognition of two recognition sequences. This unexpected mode of DNA binding and methylation has not been observed previously and sets a new precedent for a DNA methyltransferase. We also show that methylation at two recognition sequences occurs independently, and that GTWWAC motifs are enriched in intergenic regions of a strain of B. cenocepacia's genome. We further computationally assess the interactions underlying the affinities of different ligands (SAM, SAH, and sinefungin) for M.BceJIV, as a step towards developing selective inhibitors for limiting B. cenocepacia infection.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
