This is a preprint.
An optimized live imaging and growth analysis approach for Arabidopsis Sepals
- PMID: 38328103
- PMCID: PMC10849554
- DOI: 10.1101/2024.01.22.576735
An optimized live imaging and growth analysis approach for Arabidopsis Sepals
Update in
-
An optimized live imaging and multiple cell layer growth analysis approach using Arabidopsis sepals.Front Plant Sci. 2024 Sep 3;15:1449195. doi: 10.3389/fpls.2024.1449195. eCollection 2024. Front Plant Sci. 2024. PMID: 39290725 Free PMC article.
Abstract
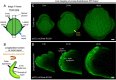
Background: Arabidopsis thaliana sepals are excellent models for analyzing growth of entire organs due to their relatively small size, which can be captured at a cellular resolution under a confocal microscope [1]. To investigate how growth of different tissue layers generates unique organ morphologies, it is necessary to live-image deep into the tissue. However, imaging deep cell layers of the sepal is practically challenging, as it is hindered by the presence of extracellular air spaces between mesophyll cells, among other factors which causes optical aberrations. Image processing is also difficult due to the low signal-to-noise ratio of the deeper tissue layers, an issue mainly associated with live imaging datasets. Addressing some of these challenges, we provide an optimized methodology for live imaging sepals and subsequent image processing. This helps us track the growth of individual cells on the outer and inner epidermal layers, which are the key drivers of sepal morphogenesis.
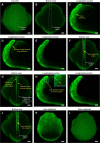
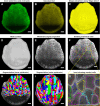
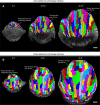
Results: For live imaging sepals across all tissue layers at early stages of development, we found that the use of a bright fluorescent membrane marker, coupled with increased laser intensity and an enhanced Z- resolution produces high-quality images suitable for downstream image processing. Our optimized parameters allowed us to image the bottommost cell layer of the sepal (inner epidermal layer) without compromising viability. We used a 'voxel removal' technique to visualize the inner epidermal layer in MorphoGraphX [2, 3] image processing software. Finally, we describe the process of optimizing the parameters for creating a 2.5D mesh surface for the inner epidermis. This allowed segmentation and parent tracking of individual cells through multiple time points, despite the weak signal of the inner epidermal cells.
Conclusion: We provide a robust pipeline for imaging and analyzing growth across inner and outer epidermal layers during early sepal development. Our approach can potentially be employed for analyzing growth of other internal cell layers of the sepals as well. For each of the steps, approaches, and parameters we used, we have provided in-depth explanations to help researchers understand the rationale and replicate our pipeline.
Figures

Similar articles
-
An optimized live imaging and multiple cell layer growth analysis approach using Arabidopsis sepals.Front Plant Sci. 2024 Sep 3;15:1449195. doi: 10.3389/fpls.2024.1449195. eCollection 2024. Front Plant Sci. 2024. PMID: 39290725 Free PMC article.
-
An optimized pipeline for live imaging whole Arabidopsis leaves at cellular resolution.Plant Methods. 2023 Feb 1;19(1):10. doi: 10.1186/s13007-023-00987-2. Plant Methods. 2023. PMID: 36726130 Free PMC article.
-
3D morphological analysis of Arabidopsis sepals.Methods Cell Biol. 2020;160:311-326. doi: 10.1016/bs.mcb.2020.03.006. Epub 2020 Apr 23. Methods Cell Biol. 2020. PMID: 32896325
-
PRESSED FLOWER works downstream of ASYMMETRIC LEAVES 2 to affect sepal flatness in Arabidopsis.J Exp Bot. 2025 May 12:eraf200. doi: 10.1093/jxb/eraf200. Online ahead of print. J Exp Bot. 2025. PMID: 40351231
-
Imagining the future of optical microscopy: everything, everywhere, all at once.Commun Biol. 2023 Oct 28;6(1):1096. doi: 10.1038/s42003-023-05468-9. Commun Biol. 2023. PMID: 37898673 Free PMC article. Review.
References
-
- Richman D.P., et al., Mechanical model of brain convolutional development. Science, 1975. 189(4196): p. 18–21. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
