This is a preprint.
Electrically silent KvS subunits associate with native Kv2 channels in brain and impact diverse properties of channel function
- PMID: 38328147
- PMCID: PMC10849721
- DOI: 10.1101/2024.01.25.577135
Electrically silent KvS subunits associate with native Kv2 channels in brain and impact diverse properties of channel function
Update in
-
The Electrically Silent Kv5.1 Subunit Forms Heteromeric Kv2 Channels in Cortical Neurons and Confers Distinct Functional Properties.J Neurosci. 2025 Mar 26;45(13):e2293232025. doi: 10.1523/JNEUROSCI.2293-23.2025. J Neurosci. 2025. PMID: 39933932 Free PMC article.
Abstract
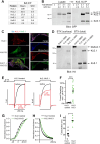
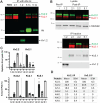
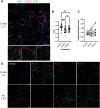
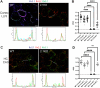
Voltage-gated K+ channels of the Kv2 family are highly expressed in brain and play dual roles in regulating neuronal excitability and in organizing endoplasmic reticulum - plasma membrane (ER-PM) junctions. Studies in heterologous cells suggest that the two pore-forming alpha subunits Kv2.1 and Kv2.2 assemble with "electrically silent" KvS subunits to form heterotetrameric channels with distinct biophysical properties. Here, using mass spectrometry-based proteomics, we identified five KvS subunits as components of native Kv2.1 channels immunopurified from mouse brain, the most abundant being Kv5.1. We found that Kv5.1 co-immunoprecipitates with Kv2.1 and to a lesser extent with Kv2.2 from brain lysates, and that Kv5.1 protein levels are decreased by 70% in Kv2.1 knockout mice and 95% in Kv2.1/2.2 double knockout mice. Multiplex immunofluorescent labelling of rodent brain sections revealed that in neocortex Kv5.1 immunolabeling is apparent in a large percentage of Kv2.1 and Kv2.2-positive layer 2/3 neurons, and in a smaller percentage of layer 5 and 6 neurons. At the subcellular level, Kv5.1 is co-clustered with Kv2.1 and Kv2.2 at ER-PM junctions in cortical neurons, although clustering of Kv5.1-containing channels is reduced relative to homomeric Kv2 channels. We also found that in heterologous cells coexpression with Kv5.1 reduces the clustering and alters the pharmacological properties of Kv2.1 channels. Together, these findings demonstrate that the Kv5.1 electrically silent subunit is a component of a substantial fraction of native brain Kv2 channels, and that its incorporation into heteromeric channels can impact diverse aspects of Kv2 channel function.
Conflict of interest statement
Conflict of interest statement: The authors declare no competing financial interests.
Figures


References
-
- Allen NM, Weckhuysen S, Gorman K, King MD, Lerche H (2020) Genetic potassium channel-associated epilepsies: Clinical review of the Kv family. Eur J Paediatr Neurol 24:105–116. - PubMed
-
- Berod A, Hartman BK, Pujol JF (1981) Importance of fixation in immunohistochemistry: use of formaldehyde solutions at variable pH for the localization of tyrosine hydroxylase. J Histochem Cytochem 29:844–850. - PubMed
-
- Bishop HI, Cobb MM, Kirmiz M, Parajuli LK, Mandikian D, Philp AM, Melnik M, Kuja-Panula J, Rauvala H, Shigemoto R, Murray KD, Trimmer JS (2018) Kv2 Ion Channels Determine the Expression and Localization of the Associated AMIGO-1 Cell Adhesion Molecule in Adult Brain Neurons. Front Mol Neurosci 11:1. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
