This is a preprint.
Blood-based screening for HPV-associated cancers
- PMID: 38328243
- PMCID: PMC10849671
- DOI: 10.1101/2024.01.04.24300841
Blood-based screening for HPV-associated cancers
Abstract
Background: HPV-associated oropharyngeal cancer (HPV+OPSCC) is the most common HPV-associated cancer in the United States yet unlike cervical cancer lacks a screening test. HPV+OPSCCs are presumed to start developing 10-15 years prior to clinical diagnosis. Circulating tumor HPV DNA (ctHPVDNA) is a sensitive and specific biomarker for HPV+OPSCC. Taken together, blood-based screening for HPV+OPSCC may be feasible years prior to diagnosis.
Methods: We developed an HPV whole genome sequencing assay, HPV-DeepSeek, with 99% sensitivity and specificity at clinical diagnosis. 28 plasma samples from HPV+OPSCC patients collected 1.3-10.8 years prior to diagnosis along with 1:1 age and gender-matched controls were run on HPV-DeepSeek and an HPV serology assay.
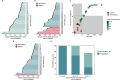
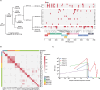
Results: 22/28 (79%) of cases and 0/28 controls screened positive for HPV+OPSCC with 100% detection within four years of diagnosis and a maximum lead time of 7.8 years. We next applied a machine learning model classifying 27/28 cases (96%) with 100% detection within 10 years. Plasma-based PIK3CA gene mutations, viral genome integration events and HPV serology were used to orthogonally validate cancer detection with 68% (19/28) of the cohort having multiple cancer signals detected. Molecular fingerprinting of HPV genomes was performed across patients demonstrating that each viral genome was unique, ruling out contamination. In patients with tumor blocks from diagnosis (15/28), molecular fingerprinting was performed within patients confirming the same viral genome across time.
Conclusions: We demonstrate accurate blood-based detection of HPV-associated cancers with lead times up to 10 years before clinical cancer diagnosis and in doing so, highlight the enormous potential of ctDNA-based cancer screening.
Conflict of interest statement
Daniel L. Faden has received research funding or in-kind funding from Bristol-Myers Squibb, Calico, Predicine, BostonGene and Neogenomics. He has received consulting fees from Merck, Noetic, Chrysalis Biomedical Advisors, Arcadia and Focus. None of these sources relate to the work in this manuscritpt.
Figures


References
-
- Liu MC, Oxnard GR, Klein EA, Swanton C, Seiden MV. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Annals of oncology : official journal of the European Society for Medical Oncology 2020;31(6):745–759. (In eng). DOI: 10.1016/j.annonc.2020.02.011. - DOI - PMC - PubMed
-
- U.S. Preventive Services Task Force, Grade A and B, Category Cancer. (https://uspreventiveservicestaskforce.org/uspstf/topic_search_results?to...).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
