Long-term benefits of dapagliflozin on renal outcomes of type 2 diabetes under routine care: a comparative effectiveness study on propensity score matched cohorts at low renal risk
- PMID: 38328413
- PMCID: PMC10847023
- DOI: 10.1016/j.lanepe.2024.100847
Long-term benefits of dapagliflozin on renal outcomes of type 2 diabetes under routine care: a comparative effectiveness study on propensity score matched cohorts at low renal risk
Abstract
Background: Despite the overall improvement in care, people with type 2 diabetes (T2D) experience an excess risk of end-stage kidney disease. We evaluated the long-term effectiveness of dapagliflozin on kidney function and albuminuria in patients with T2D.
Methods: We included patients with T2D who initiated dapagliflozin or comparators from 2015 to 2020. Propensity score matching (PSM) was performed to balance the two groups. The primary endpoint was the change in estimated glomerular filtration rate (eGFR) from baseline to the end of observation. Secondary endpoints included changes in albuminuria and loss of kidney function.
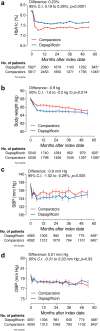
Findings: We analysed two matched groups of 6197 patients each. The comparator group included DPP-4 inhibitors (40%), GLP-1RA (22.3%), sulphonylureas (16.1%), pioglitazone (8%), metformin (5.8%), or acarbose (4%). Only 6.4% had baseline eGFR <60 ml/min/1.73 m2 and 15% had UACR >30 mg/g. During a mean follow-up of 2.5 year, eGFR declined significantly less in the dapagliflozin vs comparator group by 1.81 ml/min/1.73 m2 (95% C.I. from 1.13 to 2.48; p < 0.0001). The mean eGFR slope was significantly less negative in the dapagliflozin group by 0.67 ml/min/1.73 m2/year (95% C.I. from 0.47 to 0.88; p < 0.0001). Albuminuria declined significantly in new-users of dapagliflozin within 6 months and remained on average 44.3 mg/g lower (95% C.I. from -66.9 to -21.7; p < 0.0001) than in new-users of comparators. New-users of dapagliflozin had significantly lower rates of new-onset CKD, loss of kidney function, and a composite renal outcome. Results were confirmed for all SGLT2 inhibitors, in patients without baseline CKD, and when GLP-1RA were excluded from comparators.
Interpretation: Initiating dapagliflozin improved kidney function outcomes and albuminuria in patients with T2D and a low renal risk.
Funding: Funded by the Italian Diabetes Society and partly supported by a grant from AstraZeneca.
Keywords: Chronic kidney disease; Observational; Prevention; SGLT2 inhibitors; Type 2 diabetes.
© 2024 The Author(s).
Conflict of interest statement
GPF received fees for lectures, consultancy, or advisory board from Abbott, AstraZeneca, Boehringer, Lilly, MSD, Mundipharma, Novo Nordisk, Sanofi, Servier, Takeda. MLM received lecture or consultancy fees from AstraZeneca, Lilly, MSD, Mylan, Novo Nordisk, SlaPharma, and Servier. SDP consulted for Applied Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novartis, Novo Nordisk, and Sanofi, and received funding for these consulting services; received grant support from AstraZeneca and Boehringer Ingelheim; and received speaker fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novartis, Novo Nordisk, and Sanofi. AA received research grants, lecture, or advisory board fees from Merck Sharp & Dome, AstraZeneca, Novartis, Boeringher-Ingelheim, Sanofi, Mediolanum, Janssen, Novo Nordisk, Lilly, Servier, and Takeda. AS served on the advisory board of Novo Nordisk, Sankyo, and Sanofi and received grant support from Sankyo and speaker fees from Astra Zeneca, Bayer, Lilly, Novo Nordisk, and Sanofi. EL has nothing to disclose.
Figures




References
-
- Gregg E.W., Li Y., Wang J., et al. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med. 2014;370(16):1514–1523. - PubMed
-
- US Renal Data System (USRDS) National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2014. Annual data report: atlas of end-stage renal disease in the United States.
-
- Wen C.P., Chang C.H., Tsai M.K., et al. Diabetes with early kidney involvement may shorten life expectancy by 16 years. Kidney Int. 2017;92(2):388–396. - PubMed
-
- Neuen B.L., Young T., Heerspink H.J.L., et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7(11):845–854. - PubMed
-
- Perkovic V., Jardine M.J., Neal B., et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

