Long-term efficacy of house dust mite sublingual immunotherapy on clinical and pulmonary function in patients with asthma and allergic rhinitis
- PMID: 38328802
- PMCID: PMC10847160
- DOI: 10.1016/j.jacig.2024.100206
Long-term efficacy of house dust mite sublingual immunotherapy on clinical and pulmonary function in patients with asthma and allergic rhinitis
Abstract
Background: A previous study reported that house dust mite (HDM) sublingual immunotherapy (SLIT) for 48 weeks was effective as add-on treatment for allergic asthma; however, data regarding its long-term efficacy are scarce.
Objective: We sought to evaluate the effect of HDM SLIT on asthma control, pulmonary function, and airway inflammation and remodeling throughout the 5-year treatment period.
Methods: A total of 140 patients with asthma and allergic rhinitis sensitized to HDM were randomized to receive either drugs alone or drugs plus SLIT for 5 years. The 5-item Asthma Control Questionnaire (ACQ-5), Asthma Quality of Life Questionnaire (AQLQ), Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ), spirometry, quantitative computed tomography, and type 2 biomarkers were assessed.
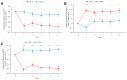
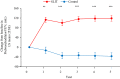
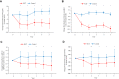
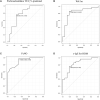
Results: An improvement in the ACQ-5, AQLQ, and RQLQ scores was observed in the SLIT group compared with the control group. HDM SLIT increased lung function and reduced the percentage of airway wall area. The levels of fractional exhaled nitric oxide (Feno), blood eosinophil, serum specific IgE for HDM, and total IgE decreased and were sustained during the 5 years. The change in type 2 biomarkers correlated with change in the AQLQ score. On the basis of receiver-operating characteristic analysis for predicting responders, the area under the receiver-operating characteristic curve in FEV1% predicted, airway wall area, Feno, and specific IgE was high. Multivariate regression analysis showed that the strongest predictor of responders was Feno.
Conclusions: HDM SLIT continued to provide sustained efficacy, improve lung function, and prevent progression of airway inflammation and remodeling in asthma throughout the 5-year treatment period.
Keywords: Airway inflammation; allergic rhinitis; asthma; house dust mite sublingual immunotherapy; long-term efficacy; pulmonary function; quality of life; remodeling; symptom; type 2 biomarker.
© 2024 The Authors.
Figures

References
-
- Jutel M., Agache I., Bonini S., Burks A.W., Calderon M., Canonica W., et al. International Consensus on Allergen Immunotherapy II: mechanisms, standardization, and pharmacoeconomics. J Allergy Clin Immunol. 2016;137:358–368. - PubMed
-
- Roberts G., Pfaar O., Akdis C.A., Ansotegui I.J., Durham S.R., Gerth van Wijk R., et al. EAACI Guidelines on Allergen Immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018;73:765–798. - PubMed
-
- Nolte H., Maloney J. The global development and clinical efficacy of sublingual tablet immunotherapy for allergic diseases. Allergol Int. 2018;67:301–308. - PubMed
-
- Calderón M.A., Linneberg A., Kleine-Tebbe J., de Blay F., Hernandez Fernandez de Rojas D., Virchow J.C., et al. Respiratory allergy caused by house dust mites: what do we really know? J Allergy Clin Immunol. 2015;136:38–48. - PubMed
LinkOut - more resources
Full Text Sources

