Efficacy and safety of transcranial magnetic stimulation on cognition in mild cognitive impairment, Alzheimer's disease, Alzheimer's disease-related dementias, and other cognitive disorders: a systematic review and meta-analysis
- PMID: 38329083
- PMCID: PMC11306417
- DOI: 10.1017/S1041610224000085
Efficacy and safety of transcranial magnetic stimulation on cognition in mild cognitive impairment, Alzheimer's disease, Alzheimer's disease-related dementias, and other cognitive disorders: a systematic review and meta-analysis
Abstract
Objective: We aim to analyze the efficacy and safety of TMS on cognition in mild cognitive impairment (MCI), Alzheimer's disease (AD), AD-related dementias, and nondementia conditions with comorbid cognitive impairment.
Design: Systematic review, Meta-Analysis.
Setting: We searched MEDLINE, Embase, Cochrane database, APA PsycINFO, Web of Science, and Scopus from January 1, 2000, to February 9, 2023.
Participants and interventions: RCTs, open-label, and case series studies reporting cognitive outcomes following TMS intervention were included.
Measurement: Cognitive and safety outcomes were measured. Cochrane Risk of Bias for RCTs and MINORS (Methodological Index for Non-Randomized Studies) criteria were used to evaluate study quality. This study was registered with PROSPERO (CRD42022326423).
Results: The systematic review included 143 studies (n = 5,800 participants) worldwide, encompassing 94 RCTs, 43 open-label prospective, 3 open-label retrospective, and 3 case series. The meta-analysis included 25 RCTs in MCI and AD. Collectively, these studies provide evidence of improved global and specific cognitive measures with TMS across diagnostic groups. Only 2 studies (among 143) reported 4 adverse events of seizures: 3 were deemed TMS unrelated and another resolved with coil repositioning. Meta-analysis showed large effect sizes on global cognition (Mini-Mental State Examination (SMD = 0.80 [0.26, 1.33], p = 0.003), Montreal Cognitive Assessment (SMD = 0.85 [0.26, 1.44], p = 0.005), Alzheimer's Disease Assessment Scale-Cognitive Subscale (SMD = -0.96 [-1.32, -0.60], p < 0.001)) in MCI and AD, although with significant heterogeneity.
Conclusion: The reviewed studies provide favorable evidence of improved cognition with TMS across all groups with cognitive impairment. TMS was safe and well tolerated with infrequent serious adverse events.
Keywords: MCI; TMS; cognition; dementia; meta-analysis; mild cognitive impairment; systematic review; transcranial magnetic stimulation.
© The Author(s), 2024. Published by Cambridge University Press on behalf of International Psychogeriatric Association.
Figures


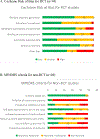
References
-
- Gauthier S, Rosa-Neto P, Morais J, Webster C. World Alzheimer Report 2021: Journey through the diagnosis of dementia. Alzheimer’s Disease International 2021.
-
- Gaugler J, Bryan James T, Reimer J, Weuve J. Alzheimer’s Association. 2021 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dementia: Chicago, IL, USA 2021; 17.
-
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in Early Alzheimer’s Disease. N Engl J Med 2022. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

