Abnormalities in microbiota/butyrate/FFAR3 signaling in aging gut impair brain function
- PMID: 38329121
- PMCID: PMC10967378
- DOI: 10.1172/jci.insight.168443
Abnormalities in microbiota/butyrate/FFAR3 signaling in aging gut impair brain function
Abstract
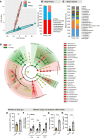
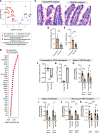
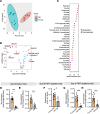
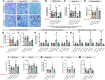
Aging-related abnormalities in gut microbiota are associated with cognitive decline, depression, and anxiety, but underlying mechanisms remain unstudied. Here, our study demonstrated that transplanting old gut microbiota to young mice induced inflammation in the gut and brain coupled with cognitive decline, depression, and anxiety. We observed diminished mucin formation and increased gut permeability ("leaky gut") with a reduction in beneficial metabolites like butyrate because of decline in butyrate-producing bacteria in the aged gut microbiota. This led to suppressed expression of butyrate receptors, free fatty acid receptors 2 and 3 (FFAR2/3). Administering butyrate alleviated inflammation, restored mucin expression and gut barriers, and corrected brain dysfunction. Furthermore, young mice with intestine-specific loss of FFAR2/3 exhibited gut and brain abnormalities akin to those in older mice. Our results demonstrate that reduced butyrate-producing bacteria in aged gut microbiota result in low butyrate levels and reduced FFAR2/3 signaling, leading to suppressed mucin formation that increases gut permeability, inflammation, and brain abnormalities. These findings underscore the significance of butyrate-FFAR2/3 agonism as a potential strategy to mitigate aged gut microbiota-induced detrimental effects on gut and brain health in older adults.
Keywords: Aging; Alzheimer disease; Bioinformatics; G protein–coupled receptors; Microbiology.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

