Sema6D forward signaling impairs T cell activation and proliferation in head and neck cancer
- PMID: 38329122
- PMCID: PMC10967380
- DOI: 10.1172/jci.insight.166349
Sema6D forward signaling impairs T cell activation and proliferation in head and neck cancer
Abstract
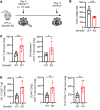
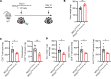
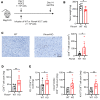
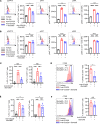
Immune checkpoint inhibitors (ICIs) are indicated for a diverse range of cancer types, and characterizing the tumor immune microenvironment is critical for optimizing therapeutic strategies, including ICIs. T cell infiltration and activation status in the tumor microenvironment greatly affects the efficacy of ICIs. Here, we show that semaphorin 6D (Sema6D) forward signaling, which is reportedly involved in coordinating the orientation of cell development and migration as a guidance factor, impaired the infiltration and activation of tumor-specific CD8+ T cells in murine oral tumors. Sema6D expressed by nonhematopoietic cells was responsible for this phenotype. Plexin-A4, a receptor for Sema6D, inhibited T cell infiltration and partially suppressed CD8+ T cell activation and proliferation induced by Sema6D stimulation. Moreover, mouse oral tumors, which are resistant to PD-1-blocking treatment in wild-type mice, showed a response to the treatment in Sema6d-KO mice. Finally, analyses of public data sets of human head and neck squamous cell carcinoma, pan-cancer cohorts, and a retrospective cohort study showed that SEMA6D was mainly expressed by nonhematopoietic cells such as cancer cells, and SEMA6D expression was significantly negatively correlated with CD8A, PDCD1, IFNG, and GZMB expression. Thus, targeting Sema6D forward signaling is a promising option for increasing ICI efficacy.
Keywords: Cancer immunotherapy; Head and neck cancer; Immunology; Oncology; T cells.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

