Blocking IL-6 signaling prevents astrocyte-induced neurodegeneration in an iPSC-based model of Parkinson's disease
- PMID: 38329129
- PMCID: PMC10967472
- DOI: 10.1172/jci.insight.163359
Blocking IL-6 signaling prevents astrocyte-induced neurodegeneration in an iPSC-based model of Parkinson's disease
Abstract
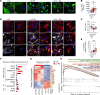
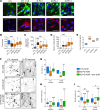
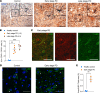
Parkinson's disease (PD) is a neurodegenerative disease associated with progressive death of midbrain dopamine (DAn) neurons in the substantia nigra (SN). Since it has been proposed that patients with PD exhibit an overall proinflammatory state, and since astrocytes are key mediators of the inflammation response in the brain, here we sought to address whether astrocyte-mediated inflammatory signaling could contribute to PD neuropathology. For this purpose, we generated astrocytes from induced pluripotent stem cells (iPSCs) representing patients with PD and healthy controls. Transcriptomic analyses identified a unique inflammatory gene expression signature in PD astrocytes compared with controls. In particular, the proinflammatory cytokine IL-6 was found to be highly expressed and released by PD astrocytes and was found to induce toxicity in DAn. Mechanistically, neuronal cell death was mediated by IL-6 receptor (IL-6R) expressed in human PD neurons, leading to downstream activation of STAT3. Blockage of IL-6R by the addition of the FDA-approved anti-IL-6R antibody, Tocilizumab, prevented PD neuronal death. SN neurons overexpressing IL-6R and reactive astrocytes expressing IL-6 were detected in postmortem brain tissue of patients at early stages of PD. Our findings highlight the potential role of astrocyte-mediated inflammatory signaling in neuronal loss in PD and pave the way for the design of future therapeutics.
Keywords: Inflammation; Neurodegeneration; Neuroscience; Parkinson disease; iPS cells.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

