Plasma biomarkers of Alzheimer's disease in the continuum of dementia with Lewy bodies
- PMID: 38329197
- PMCID: PMC11032523
- DOI: 10.1002/alz.13653
Plasma biomarkers of Alzheimer's disease in the continuum of dementia with Lewy bodies
Abstract
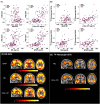
Introduction: Patients with dementia with Lewy bodies (DLB) may have Alzheimers disease (AD) pathology that can be detected by plasma biomarkers. Our objective was to evaluate plasma biomarkers of AD and their association with positron emission tomography (PET) biomarkers of amyloid and tau deposition in the continuum of DLB, starting from prodromal stages of the disease.
Methods: The cohort included patients with isolated rapid eye movement (REM) sleep behavior disorder (iRBD), mild cognitive impairment with Lewy bodies (MCI-LB), or DLB, with a concurrent blood draw and PET scans.
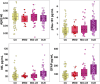
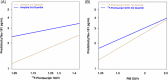
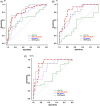
Results: Abnormal levels of plasma glial fibrillary acidic protein (GFAP) were found at the prodromal stage of MCI-LB in association with increased amyloid PET. Abnormal levels of plasma phosphorylated tau (p-tau)-181 and neurofilament light (NfL) were found at the DLB stage. Plasma p-tau-181 showed the highest accuracy in detecting abnormal amyloid and tau PET in patients with DLB.
Discussion: The range of AD co-pathology can be detected with plasma biomarkers in the DLB continuum, particularly with plasma p-tau-181 and GFAP.
Keywords: Alzheimer's disease; Lewy body; PET biomarkers; REM sleep behavior disorder; mild cognitive impairment; plasma biomarkers.
© 2024 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest. Author disclosures are available in the supporting information
Figures
References
-
- Donaghy PC, Firbank M, Petrides G, et al. The relationship between plasma biomarkers and amyloid PET in dementia with Lewy bodies. Parkinsonism Relat Disord. 2022;101:111‐116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

