The hormonal control of parturition
- PMID: 38329421
- PMCID: PMC11380996
- DOI: 10.1152/physrev.00019.2023
The hormonal control of parturition
Abstract
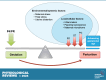
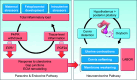
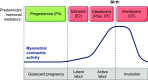
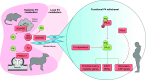
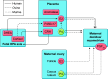
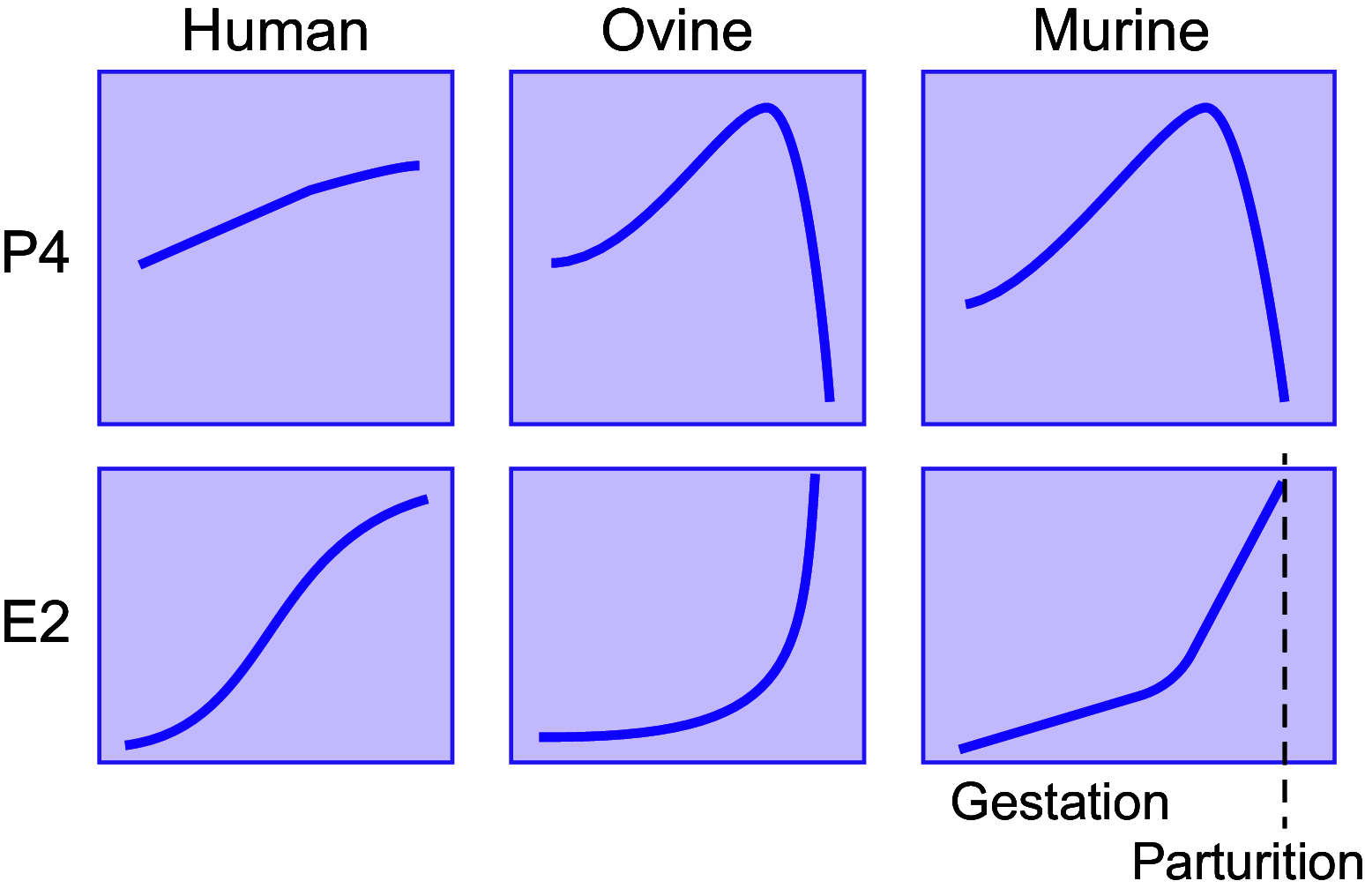
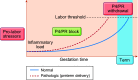
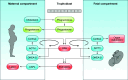
Parturition is a complex physiological process that must occur in a reliable manner and at an appropriate gestation stage to ensure a healthy newborn and mother. To this end, hormones that affect the function of the gravid uterus, especially progesterone (P4), 17β-estradiol (E2), oxytocin (OT), and prostaglandins (PGs), play pivotal roles. P4 via the nuclear P4 receptor (PR) promotes uterine quiescence and for most of pregnancy exerts a dominant block to labor. Loss of the P4 block to parturition in association with a gain in prolabor actions of E2 are key transitions in the hormonal cascade leading to parturition. P4 withdrawal can occur through various mechanisms depending on species and physiological context. Parturition in most species involves inflammation within the uterine tissues and especially at the maternal-fetal interface. Local PGs and other inflammatory mediators may initiate parturition by inducing P4 withdrawal. Withdrawal of the P4 block is coordinated with increased E2 actions to enhance uterotonic signals mediated by OT and PGs to promote uterine contractions, cervix softening, and membrane rupture, i.e., labor. This review examines recent advances in research to understand the hormonal control of parturition, with focus on the roles of P4, E2, PGs, OT, inflammatory cytokines, and placental peptide hormones together with evolutionary biology of and implications for clinical management of human parturition.
Keywords: hormonal; human; parturition; pregnancy.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Mesiano S. The endocrinology of human pregnancy and fetoplacental neuroendocrine development. In: Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management, edited by Strauss JF, Barbieri RL.. Philadelphia, PA: Elsevier, 2009, p. 249–282.
-
- Mesiano S, DeFranco E, Muglia L. Parturition. In: Knobil and Neill’s Physiology of Reproduction, edited by Plant TM, Zeleznik A.. London: Academic Press, 2014, p. 1857–1925.
-
- Casey ML, Macdonald PC. Endocrine changes of pregnancy. In: Williams Textbook of Endocrinology, edited by Wilson JD, Foster DW, Kronenberg HM, Larsen PR.. Philadelphia, PA: W.B. Saunders, 1998, p. 1259.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

