Adjuvant holmium-166 radioembolization after radiofrequency ablation in early-stage hepatocellular carcinoma patients: a dose-finding study (HORA EST HCC trial)
- PMID: 38329507
- PMCID: PMC11139702
- DOI: 10.1007/s00259-024-06630-z
Adjuvant holmium-166 radioembolization after radiofrequency ablation in early-stage hepatocellular carcinoma patients: a dose-finding study (HORA EST HCC trial)
Abstract
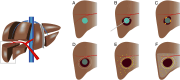
Purpose: The aim of this study was to investigate the biodistribution of (super-)selective trans-arterial radioembolization (TARE) with holmium-166 microspheres (166Ho-MS), when administered as adjuvant therapy after RFA of HCC 2-5 cm. The objective was to establish a treatment volume absorbed dose that results in an absorbed dose of ≥ 120 Gy on the hyperemic zone around the ablation necrosis (i.e., target volume).
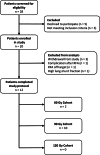
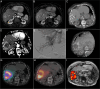
Methods: In this multicenter, prospective dose-escalation study in BCLC early stage HCC patients with lesions 2-5 cm, RFA was followed by (super-)selective infusion of 166Ho-MS on day 5-10 after RFA. Dose distribution within the treatment volume was based on SPECT-CT. Cohorts of up to 10 patients were treated with an incremental dose (60 Gy, 90 Gy, 120 Gy) of 166Ho-MS to the treatment volume. The primary endpoint was to obtain a target volume dose of ≥ 120 Gy in 9/10 patients within a cohort.
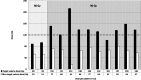
Results: Twelve patients were treated (male 10; median age, 66.5 years (IQR, [64.3-71.7])) with a median tumor diameter of 2.7 cm (IQR, [2.1-4.0]). At a treatment volume absorbed dose of 90 Gy, the primary endpoint was met with a median absorbed target volume dose of 138 Gy (IQR, [127-145]). No local recurrences were found within 1-year follow-up.
Conclusion: Adjuvant (super-)selective infusion of 166Ho-MS after RFA for the treatment of HCC can be administered safely at a dose of 90 Gy to the treatment volume while reaching a dose of ≥ 120 Gy to the target volume and may be a favorable adjuvant therapy for HCC lesions 2-5 cm.
Trial registration: Clinicaltrials.gov NCT03437382 . (registered: 19-02-2018).
Keywords: Adjuvant therapy; Dose-escalation study; Hepatocellular carcinoma; Holmium-166; Radiofrequency ablation; Trans-arterial radioembolization.
© 2024. The Author(s).
Conflict of interest statement
M.C. Burgmans has received an educational grant from Boston Scientific and Medtronic and consultancy fees from SIRTeX, Medtronic, Delcath Systems, and Philips Health Care. None are related to the current project. J.F.W. Nijsen is co-founder of Quirem Medical, which has been acquired by Terumo Europe NV in July 2020. Nijsen has a scientific advisory role and is entitled to certain milestone payments from Terumo, which are related to Quirem’s financial, operational, and regulatory performance in the future. Furthermore, Nijsen is inventor on the patents related to radioactive microspheres that are assigned to University Medical Center Utrecht Holding BV, Quirem Medical, or BASF Corp. The activities of J.F.W. Nijsen within Quirem Medical are approved and supported by the Board of Directors of the Radboudumc. All other authors declare that they have no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

