Evaluating the costs of adverse drug events in hospitalized patients: a systematic review
- PMID: 38329561
- PMCID: PMC10851489
- DOI: 10.1186/s13561-024-00481-y
Evaluating the costs of adverse drug events in hospitalized patients: a systematic review
Abstract
Background: Adverse drug events (ADEs) are not only a safety and quality of care issue for patients, but also an economic issue with significant costs. Because they often occur during hospital stays, it is necessary to accurately quantify the costs of ADEs. This review aimed to investigate the methods to calculate these costs, and to characterize their nature.
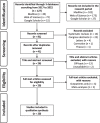
Methods: A systematic literature review was conducted to identify methods used to assess the cost of ADEs on Medline, Web of Science and Google Scholar. Original articles published from 2017 to 2022 in English and French were included. Economic evaluations were included if they concerned inpatients.
Results: From 127 studies screened, 20 studies were analyzed. There was a high heterogeneity in nature of costs, methods used, values obtained, and time horizon chosen. A small number of studies considered non-medical (10%), indirect (20%) and opportunity costs (5%). Ten different methods for assessing the cost of ADEs have been reported and nine studies did not explain how they obtained their values.
Conclusions: There is no consensus in the literature on how to assess the costs of ADEs, due to the heterogeneity of contexts and the choice of different economic perspectives. Our study adds a well-deserved overview of the existing literature that can be a solid lead for future studies and method implementation.
Trial registration: PROSPERO registration CRD42023413071.
Keywords: Adverse drug events; Costs; Health economics; Pharmacovigilance; Systematic review.
© 2024. The Author(s).
Conflict of interest statement
No author has potential conflicts of interest to disclose.
References
-
- To err is human: building a safer health system. Washington, D.C.: National Academies Press; 2000. Available from: http://www.nap.edu/catalog/9728. Cited 2022 Aug 17. - PubMed
-
- World Health Organization, WHO Patient Safety. Patient safety curriculum guide: multi-professional edition. 2011. p. 272.
-
- World Health Organization (WHO). WHO launches global effort to halve medication-related errors in 5 years. 2017. Available from: https://www.who.int/news/item/29-03-2017-who-launches-global-effort-to-h.... Cited 2022 Aug 17.
Publication types
LinkOut - more resources
Full Text Sources