Hyperbaric Oxygen Therapy and Late Local Toxic Effects in Patients With Irradiated Breast Cancer: A Randomized Clinical Trial
- PMID: 38329746
- PMCID: PMC10853873
- DOI: 10.1001/jamaoncol.2023.6776
Hyperbaric Oxygen Therapy and Late Local Toxic Effects in Patients With Irradiated Breast Cancer: A Randomized Clinical Trial
Abstract
Importance: Hyperbaric oxygen therapy (HBOT) is proposed as treatment for late local toxic effects after breast irradiation. Strong evidence of effectiveness is lacking.
Objective: To assess effectiveness of HBOT for late local toxic effects in women who received adjuvant radiotherapy for breast cancer.
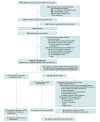
Design, setting, and participants: This was a hospital-based, pragmatic, 2-arm, randomized clinical trial nested within the prospective UMBRELLA cohort following the trials within cohorts design in the Netherlands. Participants included 189 women with patient-reported moderate or severe breast, chest wall, and/or shoulder pain in combination with mild, moderate, or severe edema, fibrosis, or movement restriction 12 months or longer after breast irradiation. Data analysis was performed from May to September 2023.
Intervention: Receipt of 30 to 40 HBOT sessions over a period of 6 to 8 consecutive weeks.
Main outcomes and measures: Breast, chest wall, and/or shoulder pain 6 months postrandomization measured by the European Organization for Research and Treatment of Cancer QLQ-BR23 questionnaire. Secondary end points were patient-reported fibrosis, edema, movement restriction, and overall quality of life. Data were analyzed according to intention-to-treat (ITT) and complier average causal effect (CACE) principles.
Results: Between November 2019 and August 2022, 125 women (median [range] age at randomization, 56 [37-85] years) with late local toxic effects were offered to undergo HBOT (intervention arm), and 61 women (median [range] age at randomization, 60 [36-80] years) were randomized to the control arm. Of those offered HBOT, 31 (25%) accepted and completed treatment. The most common reason for not accepting HBOT was high treatment intensity. In ITT, moderate or severe pain at follow-up was reported by 58 of 115 women (50%) in the intervention arm and 32 of 52 women (62%) in the control arm (odds ratio [OR], 0.63; 95% CI, 0.32-1.23; P = .18). In CACE, the proportion of women reporting moderate or severe pain at follow-up was 32% (10 of 31) among those completing HBOT and 75% (9.7 of 12.9) among control participants expected to complete HBOT if offered (adjusted OR, 0.34; 95% CI, 0.15-0.80; P = .01). In ITT, moderate or severe fibrosis was reported by 35 of 107 (33%) in the intervention arm and 25 of 49 (51%) in the control arm (OR, 0.36; 95% CI, 0.15-0.81; P = .02). There were no significant differences in breast edema, movement restriction, and quality of life between groups in ITT and CACE.
Conclusions and relevance: In this randomized clinical trial, offering HBOT to women with late local toxic effects was not effective for reducing pain, but was effective for reducing fibrosis. In the subgroup of women who completed HBOT, a significant reduction in pain and fibrosis was observed. A smaller than anticipated proportion of women with late local toxic effects was prepared to undergo HBOT.
Trial registration: ClinicalTrials.gov Identifier: NCT04193722.
Conflict of interest statement
Figures

Comment in
-
Hyperbaric Oxygen Therapy for Management of Late Radiation Toxicity-A Honey of a Trial?JAMA Oncol. 2024 Apr 1;10(4):437-438. doi: 10.1001/jamaoncol.2023.6698. JAMA Oncol. 2024. PMID: 38329763 No abstract available.
References
-
- Struikmans H, Aarts MJ, Jobsen JJ, et al. . An increased utilisation rate and better compliance to guidelines for primary radiotherapy for breast cancer from 1997 till 2008: a population-based study in the Netherlands. Radiother Oncol. 2011;100(2):320-325. doi:10.1016/j.radonc.2011.05.012 - DOI - PubMed
-
- Darby S, McGale P, Correa C, et al. ; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707-1716. doi:10.1016/S0140-6736(11)61629-2 - DOI - PMC - PubMed
-
- McGale P, Taylor C, Correa C, et al. ; EBCTCG (Early Breast Cancer Trialists’ Collaborative Group) . Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127-2135. doi:10.1016/S0140-6736(14)60488-8 - DOI - PMC - PubMed
-
- Bentzen SM, Agrawal RK, Aird EG, et al. ; START Trialists’ Group . The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371(9618):1098-1107. doi:10.1016/S0140-6736(08)60348-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

