Retinoic acid receptor α activity in proximal tubules prevents kidney injury and fibrosis
- PMID: 38330015
- PMCID: PMC10873609
- DOI: 10.1073/pnas.2311803121
Retinoic acid receptor α activity in proximal tubules prevents kidney injury and fibrosis
Abstract
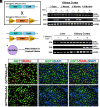
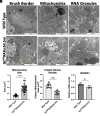
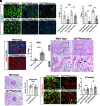
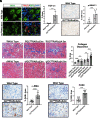
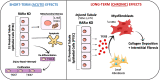
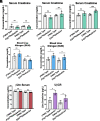
Chronic kidney disease (CKD) is characterized by a gradual loss of kidney function and affects ~13.4% of the global population. Progressive tubulointerstitial fibrosis, driven in part by proximal tubule (PT) damage, is a hallmark of late stages of CKD and contributes to the development of kidney failure, for which there are limited treatment options. Normal kidney development requires signaling by vitamin A (retinol), which is metabolized to retinoic acid (RA), an endogenous agonist for the RA receptors (RARα, β, γ). RARα levels are decreased in a mouse model of diabetic nephropathy and restored with RA administration; additionally, RA treatment reduced fibrosis. We developed a mouse model in which a spatiotemporal (tamoxifen-inducible) deletion of RARα in kidney PT cells of adult mice causes mitochondrial dysfunction, massive PT injury, and apoptosis without the use of additional nephrotoxic substances. Long-term effects (3 to 4.5 mo) of RARα deletion include increased PT secretion of transforming growth factor β1, inflammation, interstitial fibrosis, and decreased kidney function, all of which are major features of human CKD. Therefore, RARα's actions in PTs are crucial for PT homeostasis, and loss of RARα causes injury and a key CKD phenotype.
Keywords: fibrosis; kidney disease; mitochondria; proximal tubule; retinoic acid.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

Similar articles
-
Myofibroblasts acquire retinoic acid-producing ability during fibroblast-to-myofibroblast transition following kidney injury.Kidney Int. 2019 Mar;95(3):526-539. doi: 10.1016/j.kint.2018.10.017. Epub 2019 Jan 17. Kidney Int. 2019. PMID: 30661714
-
The potential signaling pathway between peroxisome proliferator-activated receptor gamma and retinoic acid receptor alpha in renal interstitial fibrosis disease.J Recept Signal Transduct Res. 2015;35(4):258-68. doi: 10.3109/10799893.2014.975249. Epub 2014 Oct 31. J Recept Signal Transduct Res. 2015. PMID: 25359573
-
Dicer deficiency in proximal tubules exacerbates renal injury and tubulointerstitial fibrosis and upregulates Smad2/3.Am J Physiol Renal Physiol. 2018 Dec 1;315(6):F1822-F1832. doi: 10.1152/ajprenal.00402.2018. Epub 2018 Oct 3. Am J Physiol Renal Physiol. 2018. PMID: 30280598 Free PMC article.
-
Disparate roles of retinoid acid signaling molecules in kidney disease.Am J Physiol Renal Physiol. 2021 May 1;320(5):F683-F692. doi: 10.1152/ajprenal.00045.2021. Epub 2021 Mar 1. Am J Physiol Renal Physiol. 2021. PMID: 33645319 Free PMC article. Review.
-
Post-translational modification of retinoic acid receptor alpha and its roles in tumor cell differentiation.Biochem Pharmacol. 2020 Jan;171:113696. doi: 10.1016/j.bcp.2019.113696. Epub 2019 Nov 11. Biochem Pharmacol. 2020. PMID: 31726045 Review.
Cited by
-
Bibliometric analysis of research on retinoic acid in the field of kidney disorders.Front Pharmacol. 2024 Aug 15;15:1435889. doi: 10.3389/fphar.2024.1435889. eCollection 2024. Front Pharmacol. 2024. PMID: 39211779 Free PMC article. Review.
-
ATF4 in proximal tubules modulates kidney function and modifies the metabolome.J Mol Med (Berl). 2025 Aug;103(8):989-1003. doi: 10.1007/s00109-025-02559-4. Epub 2025 Jun 21. J Mol Med (Berl). 2025. PMID: 40542844 Free PMC article.
-
Vitamin A is associated with all-cause mortality in patients with chronic kidney disease: a population-based cohort study.Front Nutr. 2024 Dec 4;11:1469844. doi: 10.3389/fnut.2024.1469844. eCollection 2024. Front Nutr. 2024. PMID: 39698241 Free PMC article.
-
Deletion of the transcription factor ATF4 in a model of clear cell renal cell carcinoma.Neoplasia. 2025 Aug;66:101188. doi: 10.1016/j.neo.2025.101188. Epub 2025 Jun 4. Neoplasia. 2025. PMID: 40472739 Free PMC article.
-
Elevation of ISG15 promotes diabetic kidney disease by modulating renal tubular epithelial cell pyroptosis.Clin Transl Med. 2025 Jun;15(6):e70337. doi: 10.1002/ctm2.70337. Clin Transl Med. 2025. PMID: 40462493 Free PMC article.
References
-
- Webster A. C., Nagler E. V., Morton R. L., Masson P., Chronic kidney disease. Lancet 389, 1238–1252 (2017). - PubMed
-
- Cavanaugh K. L., Diabetes management issues for patients with chronic kidney disease. Clin. Diabetes 25, 90–97 (2007).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

