Radiopacity Enhancements in Polymeric Implant Biomaterials: A Comprehensive Literature Review
- PMID: 38330191
- PMCID: PMC10934286
- DOI: 10.1021/acsbiomaterials.3c01667
Radiopacity Enhancements in Polymeric Implant Biomaterials: A Comprehensive Literature Review
Abstract
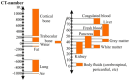
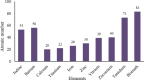
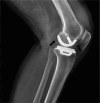
Polymers as biomaterials possess favorable properties, which include corrosion resistance, light weight, biocompatibility, ease of processing, low cost, and an ability to be easily tailored to meet specific applications. However, their inherent low X-ray attenuation, resulting from the low atomic numbers of their constituent elements, i.e., hydrogen (1), carbon (6), nitrogen (7), and oxygen (8), makes them difficult to visualize radiographically. Imparting radiopacity to radiolucent polymeric implants is necessary to enable noninvasive evaluation of implantable medical devices using conventional imaging methods. Numerous studies have undertaken this by blending various polymers with contrast agents consisting of heavy elements. The selection of an appropriate contrast agent is important, primarily to ensure that it does not cause detrimental effects to the relevant mechanical and physical properties of the polymer depending upon the intended application. Furthermore, its biocompatibility with adjacent tissues and its excretion from the body require thorough evaluation. We aimed to summarize the current knowledge on contrast agents incorporated into synthetic polymers in the context of implantable medical devices. While a single review was found that discussed radiopacity in polymeric biomaterials, the publication is outdated and does not address contemporary polymers employed in implant applications. Our review provides an up-to-date overview of contrast agents incorporated into synthetic medical polymers, encompassing both temporary and permanent implants. We expect that our results will significantly inform and guide the strategic selection of contrast agents, considering the specific requirements of implantable polymeric medical devices.
Keywords: biocompatibility; contrast agent; implant; polymers; radiolucent; radiopacity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Center for Devices and Radiological Health. Implants and Prosthetics; FDA. Online https://www.fda.gov/medical-devices/products-and-medical-procedures/impl... (accessed August 18, 2023).
-
- Al-Shalawi F. D.; Mohamed Ariff A. H.; Jung D.-W.; Mohd Ariffin M. K. A.; Seng Kim C. L.; Brabazon D.; Al-Osaimi M. O. Biomaterials as Implants in the Orthopedic Field for Regenerative Medicine: Metal versus Synthetic Polymers. Polymers 2023, 15 (12), 2601. 10.3390/polym15122601. - DOI - PMC - PubMed
-
- Vdrit K.; Dinesh K. R.. Polymers Used as Implant Biomaterials: A Review. March 2018.
-
- Marjanović-Balaban Ž.; Jelić D.. Polymeric Biomaterials in Clinical Practice. In Biomaterials in Clinical Practice: Advances in Clinical Research and Medical Devices; Zivic F., Affatato S., Trajanovic M., Schnabelrauch M., Grujovic N., Choy K. L., Eds.; Springer International Publishing: Cham, 2018; pp 101–117, 10.1007/978-3-319-68025-5_4. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
