From Basic Principles of Protein-Polysaccharide Association to the Rational Design of Thermally Sensitive Materials
- PMID: 38330192
- PMCID: PMC10895586
- DOI: 10.1021/acsami.3c12926
From Basic Principles of Protein-Polysaccharide Association to the Rational Design of Thermally Sensitive Materials
Abstract
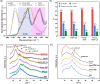
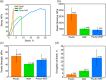
Biology resolves design requirements toward functional materials by creating nanostructured composites, where individual components are combined to maximize the macroscale material performance. A major challenge in utilizing such design principles is the trade-off between the preservation of individual component properties and emerging composite functionalities. Here, polysaccharide pectin and silk fibroin were investigated in their composite form with pectin as a thermal-responsive ion conductor and fibroin with exceptional mechanical strength. We show that segregative phase separation occurs upon mixing, and within a limited compositional range, domains ∼50 nm in size are formed and distributed homogeneously so that decent matrix collective properties are established. The composite is characterized by slight conformational changes in the silk domains, sequestering the hydrogen-bonded β-sheets as well as the emergence of randomized pectin orientations. However, most dominant in the composite's properties is the introduction of dense domain interfaces, leading to increased hydration, surface hydrophilicity, and increased strain of the composite material. Using controlled surface charging in X-ray photoelectron spectroscopy, we further demonstrate Ca ions (Ca2+) diffusion in the pectin domains, with which the fingerprints of interactions at domain interfaces are revealed. Both the thermal response and the electrical conductance were found to be strongly dependent on the degree of composite hydration. Our results provide a fundamental understanding of the role of interfacial interactions and their potential applications in the design of material properties, polysaccharide-protein composites in particular.
Keywords: biomaterials; pectin; protein nanofibrils; self-assembly; silk protein; thermal induced conductivity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Zhang M.; Sun C.; Li Q. Interaction between the Polysaccharides and Proteins in Semisolid Food Systems. Encycl. Food Chem. 2018, 439–445. 10.1016/B978-0-08-100596-5.21474-1. - DOI
-
- Wahab I. F.; Razak S. I. A. Polysaccharides as Composite Biomaterials. Compos. Renewable Sustainable Mater. 2016, 65–84. 10.5772/65263. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

