Safety and Efficacy of Apitegromab in Patients With Spinal Muscular Atrophy Types 2 and 3: The Phase 2 TOPAZ Study
- PMID: 38330285
- PMCID: PMC11067700
- DOI: 10.1212/WNL.0000000000209151
Safety and Efficacy of Apitegromab in Patients With Spinal Muscular Atrophy Types 2 and 3: The Phase 2 TOPAZ Study
Erratum in
-
Safety and Efficacy of Apitegromab in Patients With Spinal Muscular Atrophy Types 2 and 3: The Phase 2 TOPAZ Study.Neurology. 2024 Jul 9;103(1):e209519. doi: 10.1212/WNL.0000000000209519. Epub 2024 Jun 3. Neurology. 2024. PMID: 38830133 Free PMC article. No abstract available.
-
Corrections to Null Hypothesis Articles.Neurology. 2025 May 13;104(9):e213475. doi: 10.1212/WNL.0000000000213475. Epub 2025 Apr 4. Neurology. 2025. PMID: 40184595 Free PMC article. No abstract available.
Abstract
Background and objectives: Currently approved therapies for spinal muscular atrophy (SMA) reverse the degenerative course, leading to better functional outcome, but they do not address the impairment arising from preexisting neurodegeneration. Apitegromab, an investigational, fully human monoclonal antibody, inhibits activation of myostatin (a negative regulator of skeletal muscle growth), thereby preserving muscle mass. The phase 2 TOPAZ trial assessed the safety and efficacy of apitegromab in individuals with later-onset type 2 and type 3 SMA.
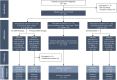
Methods: In this study, designed to investigate potential meaningful combinations of eligibility and treatment regimen for future studies, participants aged 2-21 years received IV apitegromab infusions every 4 weeks for 12 months in 1 of 3 cohorts. Cohort 1 stratified ambulatory participants aged 5-21 years into 2 arms (apitegromab 20 mg/kg alone or in combination with nusinersen); cohort 2 evaluated apitegromab 20 mg/kg combined with nusinersen in nonambulatory participants aged 5-21 years; and cohort 3 blindly evaluated 2 randomized apitegromab doses (2 and 20 mg/kg) combined with nusinersen in younger participants ≥2 years of age. The primary efficacy measure was mean change from baseline using the Hammersmith Functional Motor Scale version appropriate for each cohort. Data were analyzed using a paired t test with 2-sided 5% type 1 error for the mean change from baseline for predefined cohort-specific primary efficacy end points.
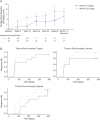
Results: Fifty-eight participants (mean age 9.4 years) were enrolled at 16 trial sites in the United States and Europe. Participants had been treated with nusinersen for a mean of 25.9 months before enrollment in any of the 3 trial cohorts. At month 12, the mean change from baseline in Hammersmith scale score was -0.3 points (95% CI -2.1 to 1.4) in cohort 1 (n = 23), 0.6 points (-1.4 to 2.7) in cohort 2 (n = 15), and in cohort 3 (n = 20), the mean scores were 5.3 (-1.5 to 12.2) and 7.1 (1.8 to 12.5) for the 2-mg/kg (n = 8) and 20-mg/kg (n = 9) arms, respectively. The 5 most frequently reported treatment-emergent adverse events were headache (24.1%), pyrexia (22.4%), upper respiratory tract infection (22.4%), cough (22.4%), and nasopharyngitis (20.7%). No deaths or serious adverse reactions were reported.
Discussion: Apitegromab led to improved motor function in participants with later-onset types 2 and 3 SMA. These results support a randomized, placebo-controlled phase 3 trial of apitegromab in participants with SMA.
Trial registration information: This trial is registered with ClinicalTrials.gov (NCT03921528).
Classification of evidence: This study provides Class III evidence that apitegromab improves motor function in later-onset types 2 and 3 spinal muscular atrophy.
Figures

References
-
- Albrechtsen SS, Born AP, Boesen MS. Nusinersen treatment of spinal muscular atrophy: a systematic review. Dan Med J. 2020;67(9):A02200100. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
