Macrophage Tim-3 maintains intestinal homeostasis in DSS-induced colitis by suppressing neutrophil necroptosis
- PMID: 38330550
- PMCID: PMC10865407
- DOI: 10.1016/j.redox.2024.103072
Macrophage Tim-3 maintains intestinal homeostasis in DSS-induced colitis by suppressing neutrophil necroptosis
Abstract
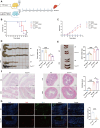
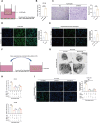
T-cell immunoglobulin domain and mucin domain-3 (Tim-3) is a versatile immunomodulator that protects against intestinal inflammation. Necroptosis is a type of cell death that regulates intestinal homeostasis and inflammation. The mechanism(s) underlying the protective role of macrophage Tim-3 in intestinal inflammation is unclear; thus, we investigated whether specific Tim-3 knockdown in macrophages drives intestinal inflammation via necroptosis. Tim-3 protein and mRNA expression were assessed via double immunofluorescence staining and single-cell RNA sequencing (sc-RNA seq), respectively, in the colonic tissues of patients with inflammatory bowel disease (IBD) and healthy controls. Macrophage-specific Tim3-knockout (Tim-3M-KO) mice were generated to explore the function and mechanism of Tim-3 in dextran sodium sulfate (DSS)-induced colitis. Necroptosis was blocked by pharmacological inhibitors of receptor-interacting protein kinase (RIP)1, RIP3, and reactive oxygen species (ROS). Additionally, in vitro experiments were performed to assess the mechanisms of neutrophil necroptosis induced by Tim-3 knockdown macrophages. Although Tim-3 is relatively inactive in macrophages during colon homeostasis, it is highly active during colitis. Compared to those in controls, Tim-3M-KO mice showed increased susceptibility to colitis, higher colitis scores, and increased pro-inflammatory mediator expression. Following the administration of RIP1/RIP3 or ROS inhibitors, a significant reduction in intestinal inflammation symptoms was observed in DSS-treated Tim-3M-KO mice. Further analysis indicated the TLR4/NF-κB pathway in Tim-3 knockdown macrophages mediates the TNF-α-induced necroptosis pathway in neutrophils. Macrophage Tim-3 regulates neutrophil necroptosis via intracellular ROS signaling. Tim-3 knockdown macrophages can recruit neutrophils and induce neutrophil necroptosis, thereby damaging the intestinal mucosal barrier and triggering a vicious cycle in the development of colitis. Our results demonstrate a protective role of macrophage Tim-3 in maintaining gut homeostasis by inhibiting neutrophil necroptosis and provide novel insights into the pathogenesis of IBD.
Keywords: Inflammatory bowel disease; Macrophage; Necroptosis; Neutrophil; ROS; Tim-3.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures













References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

