Discovery of small molecule benzothiazole and indole derivatives tackling tau 2N4R and α-synuclein fibrils
- PMID: 38330847
- PMCID: PMC10921547
- DOI: 10.1016/j.bmc.2024.117613
Discovery of small molecule benzothiazole and indole derivatives tackling tau 2N4R and α-synuclein fibrils
Abstract
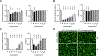
Tau and α-synuclein aggregates are the main histopathological hallmarks present in Alzheimer's disease (AD), Parkinson's disease (PD), and other neurodegenerative disorders. Intraneuronal hyperphosphorylated tau accumulation is significantly connected to the degree of cognitive impairment in AD patients. In particular, the longest 2N4R tau isoform has a propensity to rapidly form oligomers and mature fibrils. On the other hand, misfolding of α-synuclein (α-syn) is the characteristic feature in PD and dementia with Lewy bodies (DLB). There is a strong crosstalk between the two prone-to-aggregation proteins as they coprecipitated in some brains of AD, PD, and DLB patients. Simultaneous targeting of both proteinaceous oligomers and aggregates is still challenging. Here, we rationally designed and synthesized benzothiazole- and indole-based compounds using the structural hybridization strategy between the benzothiazole N744 cyanine dye and the diphenyl pyrazole Anle138b that showed anti-aggregation activity towards 2N4R tau and α-syn, respectively. The anti-aggregation effect of the prepared compounds was monitored using the thioflavin-T (ThT) fluorescence assay, while transmission electron microscopy (TEM) was employed to detect fibrils upon the completion of a time-course study with the ThT assay. Moreover, the photo-induced crosslinking of unmodified protein (PICUP) assay was used to determine the formation of oligomers. Specifically, compounds 46 and 48 demonstrated the highest anti-aggregation activity by decreasing the ThT fluorescence to 4.0 and 14.8%, respectively, against α-syn. Although no noticeable effect on 2N4R tau oligomers, 46 showed promising anti-oligomer activity against α-syn. Both compounds induced a significantly high anti-aggregation effect against the two protein fibrils as visualized by TEM. Moreover, compound 48 remarkably inhibited α-syn inclusion and cell confluence using M17D cells. Collectively, compounds 46 and 48 could serve as a basic structure for further optimization to develop clinically active AD and PD disease-modifying agents.
Keywords: Alpha-synuclein; Alzheimer’s disease; Anti-oligomer agents; Parkinson’s disease; Tau isoforms.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



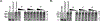


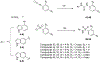
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

