Identification of a transcription factor AoMsn2 of the Hog1 signaling pathway contributes to fungal growth, development and pathogenicity in Arthrobotrys oligospora
- PMID: 38331317
- PMCID: PMC11785577
- DOI: 10.1016/j.jare.2024.02.002
Identification of a transcription factor AoMsn2 of the Hog1 signaling pathway contributes to fungal growth, development and pathogenicity in Arthrobotrys oligospora
Abstract
Introduction: Arthrobotrys oligospora has been utilized as a model strain to study the interaction between fungi and nematodes owing to its ability to capture nematodes by developing specialized traps. A previous study showed that high-osmolarity glycerol (Hog1) signaling regulates the osmoregulation and nematocidal activity of A. oligospora. However, the function of downstream transcription factors of the Hog1 signaling in the nematode-trapping (NT) fungi remains unclear.
Objective: This study aimed to investigate the functions and potential regulatory network of AoMsn2, a downstream transcription factor of the Hog1 signaling pathway in A. oligospora.
Methods: The function of AoMsn2 was characterized using targeted gene deletion, phenotypic experiments, real-time quantitative PCR, RNA sequencing, untargeted metabolomics, and yeast two-hybrid analysis.
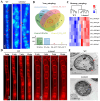
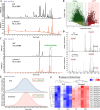
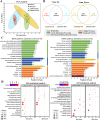
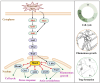
Results: Loss of Aomsn2 significantly enlarged and swollen the hyphae, with an increase in septa and a significant decrease in nuclei. In particular, spore yield, spore germination rate, traps, and nematode predation efficiency were remarkably decreased in the mutants. Phenotypic and transcriptomic analyses revealed that AoMsn2 is essential for fatty acid metabolism and autophagic pathways. Additionally, untargeted metabolomic analysis identified an important function of AoMsn2 in the modulation of secondary metabolites. Furtherly, we analyzed the protein interaction network of AoMsn2 based on the Kyoto Encyclopedia of Genes and Genomes pathway map and the online website STRING. Finally, Hog1 and six putative targeted proteins of AoMsn2 were identified by Y2H analysis.
Conclusion: Our study reveals that AoMsn2 plays crucial roles in the growth, conidiation, trap development, fatty acid metabolism, and secondary metabolism, as well as establishes a broad basis for understanding the regulatory mechanisms of trap morphogenesis and environmental adaptation in NT fungi.
Keywords: Arthrobotrys oligospora; Conidiation; Hog1 signaling pathway; Secondary metabolism; Transcription factor Msn2; Trap formation.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Li J., Zou C., Xu J., Ji X., Niu X., Yang J., et al. Molecular mechanisms of nematode-nematophagous microbe interactions: basis for biological control of plant-parasitic nematodes. Annu Rev Phytopathol. 2015;53(1):67–95. - PubMed
-
- Ahmad G., Khan A., Khan A.A., Ali A., Mohhamad H.I. Biological control: a novel strategy for the control of the plant parasitic nematodes. Antonie Van Leeuwenhoek. 2021;114(7):885–912. - PubMed
-
- Su H., Zhao Y., Zhou J., Feng H., Jiang D., Zhang K.-Q., et al. Trapping devices of nematode-trapping fungi: formation, evolution, and genomic perspectives. Biol Rev Camb Philos Soc. 2017;92(1):357–368. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

