Effects of upadacitinib on enthesitis in patients with psoriatic arthritis: a post hoc analysis of SELECT-PsA 1 and 2 trials
- PMID: 38331400
- PMCID: PMC11534117
- DOI: 10.1093/rheumatology/keae057
Effects of upadacitinib on enthesitis in patients with psoriatic arthritis: a post hoc analysis of SELECT-PsA 1 and 2 trials
Abstract
Objectives: To characterize the effect of upadacitinib 15 mg once daily (UPA15) on enthesitis in patients with PsA from the SELECT-PsA Phase 3 trials.
Methods: Patients with an inadequate response/intolerance to one or more non-biologic DMARD (SELECT-PsA 1) or one or more biologic DMARD (SELECT-PsA 2) received UPA15, adalimumab 40 mg every other week or placebo (weeks 0-24) switched to UPA15 (week 24 onward). The Leeds Enthesitis Index (LEI) and Spondyloarthritis Research Consortium of Canada (SPARCC) index were used to assess improvement in enthesitis, enthesitis resolution, maintenance of enthesitis resolution and protection from enthesitis development through week 56.
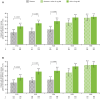
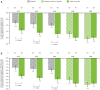
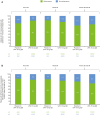
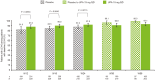
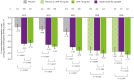
Results: Data from 639 patients receiving UPA15 and 635 patients receiving placebo (including 317 patients who switched from placebo to UPA15) were analysed. UPA15 led to higher rates of enthesitis resolution vs placebo at week 24 (LEI: 59.8% vs 38.0%; SPARCC index: 50.6% vs 31.5%, respectively) and greater improvements in the LEI (-1.7 vs -1.0) and SPARCC index (-3.4 vs -1.9); improvements were maintained through week 56. Improvements were observed after 12 weeks of UPA15 treatment. Over 90% of patients without enthesitis (LEI = 0) at baseline receiving UPA15 were enthesitis-free at week 56, and UPA15 prevented recurrence of enthesitis at week 56 in >80% of patients with enthesitis at baseline who achieved resolution (LEI = 0) at week 24.
Conclusions: UPA15 is associated with a comprehensive improvement in enthesitis, with improvements observed after 12 weeks of treatment. Additionally, treatment with UPA15 was associated with maintaining an enthesitis-free state after resolution and protection against new-onset enthesitis.
Trial registration: ClinicalTrials.gov identifiers: NCT03104400 (SELECT-PsA 1) and NCT03104374 (SELECT-PsA 2).
Keywords: Janus kinase inhibitor; entheses; enthesitis; pain; psoriatic arthritis; upadacitinib.
© The Author(s) 2024. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures

References
-
- Mease PJ, Karki C, Palmer JB et al. Clinical characteristics, disease activity, and patient-reported outcomes in psoriatic arthritis patients with dactylitis or enthesitis: results from the Corrona Psoriatic Arthritis/Spondyloarthritis Registry. Arthritis Care Res (Hoboken) 2017;69:1692–9. - PubMed
-
- Pittam B, Gupta S, Harrison NL et al. Prevalence of extra-articular manifestations in psoriatic arthritis: a systematic review and meta-analysis. Rheumatology (Oxford) 2020;59:2199–206. - PubMed
-
- Mease PJ, Liu M, Rebello S et al. Disease characteristics, quality of life, and work productivity by enthesitis site: real-world data from the US Corrona Psoriatic Arthritis/Spondyloarthritis Registry. J Rheumatol 2021;48:367–75. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

