Advances in the Structural and Functional Understanding of m1A RNA Modification
- PMID: 38331425
- PMCID: PMC10882958
- DOI: 10.1021/acs.accounts.3c00568
Advances in the Structural and Functional Understanding of m1A RNA Modification
Abstract
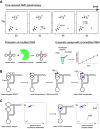
ConspectusRNA modification is a co- or post-transcriptional process by which specific nucleotides are chemically altered by enzymes after their initial incorporation into the RNA chain, expanding the chemical and functional diversity of RNAs. Our understanding of RNA modifications has changed dramatically in recent years. In the past decade, RNA methyltransferases (MTases) have been highlighted in numerous clinical studies and disease models, modifications have been found to be dynamically regulated by demodification enzymes, and significant technological advances have been made in the fields of RNA sequencing, mass spectrometry, and structural biology. Among RNAs, transfer RNAs (tRNAs) exhibit the greatest diversity and density of post-transcriptional modifications, which allow for potential cross-talks and regulation during their incorporation. N1-methyladenosine (m1A) modification is found in tRNAs at positions 9, 14, 16, 22, 57, and 58, depending on the tRNA and organism.Our laboratory has used and developed a large panel of tools to decipher the different mechanisms used by m1A tRNA MTases to recognize and methylate tRNA. We have solved the structures of TrmI from Thermus thermophilus (m1A58), TrmK from Bacillus subtilis (m1A22), and human TRMT10C (m1A9). These MTases do not share the same structure or organization to recognize tRNAs, but they all modify an adenosine, forming a non-Watson-Crick (WC) interaction. For TrmK, nuclear magnetic resonance (NMR) chemical shift mapping of the binding interface between TrmK and tRNASer was invaluable to build a TrmK/tRNA model, where both domains of TrmK participate in the binding of a full-length L-shaped tRNA and where the non-WC purine 13-A22 base pair positions the A22 N1-atom close to the methyl of the S-adenosyl-l-methionine (SAM) TrmK cofactor. For TRMT10C, cryoEM structures showed the MTase poised to N1-methylate A9 or G9 in tRNA and revealed different steps of tRNA maturation, where TRMT10C acts as a tRNA binding platform for sequential docking of each maturation enzyme. This work confers a role for TRMT10C in tRNA quality control and provides a framework to understand the link between mitochondrial tRNA maturation dysfunction and diseases.Methods to directly detect the incorporation of modifications during tRNA biosynthesis are rare and do not provide easy access to the temporality of their introduction. To this end, we have introduced time-resolved NMR to monitor tRNA maturation in the cellular environment. Combined with genetic and biochemical approaches involving the synthesis of specifically modified tRNAs, our methodology revealed that some modifications are incorporated in a defined sequential order, controlled by cross-talks between modification events. In particular, a strong modification circuit, namely Ψ55 → m5U54 → m1A58, controls the modification process in the T-arm of yeast elongator tRNAs. Conversely, we showed that m1A58 is efficiently introduced on unmodified initiator tRNAiMet without the need of any prior modification. Two distinct pathways are therefore followed for m1A58 incorporation in elongator and initiator tRNAs.We are undoubtedly entering an exciting period for the elucidation of the functions of RNA modifications and the intricate mechanisms by which modification enzymes identify and alter their RNA substrates. These are promising directions for the field of epitranscriptomics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Dégut C.; Roovers M.; Barraud P.; Brachet F.; Feller A.; Larue V.; Al Refaii A.; Caillet J.; Droogmans L.; Tisné C. Structural Characterization of B. Subtilis M1A22 TRNA Methyltransferase TrmK: Insights into TRNA Recognition. Nucleic Acids Res. 2019, 47, 4736–4750. 10.1093/nar/gkz230. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

