Azetidines Kill Multidrug-Resistant Mycobacterium tuberculosis without Detectable Resistance by Blocking Mycolate Assembly
- PMID: 38331432
- PMCID: PMC10895678
- DOI: 10.1021/acs.jmedchem.3c01643
Azetidines Kill Multidrug-Resistant Mycobacterium tuberculosis without Detectable Resistance by Blocking Mycolate Assembly
Abstract
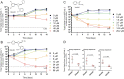
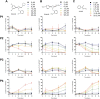
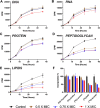
Tuberculosis (TB) is the leading cause of global morbidity and mortality resulting from infectious disease, with over 10.6 million new cases and 1.4 million deaths in 2021. This global emergency is exacerbated by the emergence of multidrug-resistant MDR-TB and extensively drug-resistant XDR-TB; therefore, new drugs and new drug targets are urgently required. From a whole cell phenotypic screen, a series of azetidines derivatives termed BGAz, which elicit potent bactericidal activity with MIC99 values <10 μM against drug-sensitive Mycobacterium tuberculosis and MDR-TB, were identified. These compounds demonstrate no detectable drug resistance. The mode of action and target deconvolution studies suggest that these compounds inhibit mycobacterial growth by interfering with cell envelope biogenesis, specifically late-stage mycolic acid biosynthesis. Transcriptomic analysis demonstrates that the BGAz compounds tested display a mode of action distinct from the existing mycobacterial cell wall inhibitors. In addition, the compounds tested exhibit toxicological and PK/PD profiles that pave the way for their development as antitubercular chemotherapies.
Conflict of interest statement
The authors declare the following competing financial interest(s): A patent application disclosing aspects of this study has been filed by the University of Birmingham. The views expressed in this publication are those of the authors and not necessarily those of Public Health England (UKHSA), or the Department of Health. The authors declare no other competing interests.
Figures




Similar articles
-
Antitubercular activity of disulfiram, an antialcoholism drug, against multidrug- and extensively drug-resistant Mycobacterium tuberculosis isolates.Antimicrob Agents Chemother. 2012 Aug;56(8):4140-5. doi: 10.1128/AAC.06445-11. Epub 2012 May 21. Antimicrob Agents Chemother. 2012. PMID: 22615274 Free PMC article.
-
High fluoroquinolone resistance proportions among multidrug-resistant tuberculosis driven by dominant L2 Mycobacterium tuberculosis clones in the Mumbai Metropolitan Region.Genome Med. 2022 Aug 22;14(1):95. doi: 10.1186/s13073-022-01076-0. Genome Med. 2022. PMID: 35989319 Free PMC article.
-
Whole-genome sequencing of clinical isolates from tuberculosis patients in India: real-world data indicates a high proportion of pre-XDR cases.Microbiol Spectr. 2024 May 2;12(5):e0277023. doi: 10.1128/spectrum.02770-23. Epub 2024 Apr 10. Microbiol Spectr. 2024. PMID: 38597637 Free PMC article.
-
Epidemiology of extensively drug-resistant tuberculosis among patients with multidrug-resistant tuberculosis: A systematic review and meta-analysis.Int J Infect Dis. 2023 Jul;132:50-63. doi: 10.1016/j.ijid.2023.04.392. Epub 2023 Apr 16. Int J Infect Dis. 2023. PMID: 37072053 Free PMC article.
-
Mycobacterium tuberculosis inhibitors: an updated patent review (2021-present).Expert Opin Ther Pat. 2024 Dec;34(12):1215-1230. doi: 10.1080/13543776.2024.2419826. Epub 2024 Nov 18. Expert Opin Ther Pat. 2024. PMID: 39431728 Review.
Cited by
-
Densely Functionalized 2-Methylideneazetidines: Evaluation as Antibacterials.Molecules. 2021 Jun 25;26(13):3891. doi: 10.3390/molecules26133891. Molecules. 2021. PMID: 34202191 Free PMC article.
References
-
- www.who.int/gho/tb/en/. World Health Organisation: Global Health Observatory (GHO) data www.who.int/gho/tb/en/.
-
- Global tuberculosis report 2022; World Health Organization: Geneva, 2022, Licence: CC BY-NC-SA 3.0 IGO.
-
- Shah N. S.; Wright A.; Bai G. H.; Barrera L.; Boulahbal F.; Martin-Casabona N.; Drobniewski F.; Gilpin C.; Havelkova M.; Lepe R.; Lumb R.; Metchock B.; Portaels F.; Rodrigues M. F.; Rusch-Gerdes S.; Van Deun A.; Vincent V.; Laserson K.; Wells C.; Cegielski J. P. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg. Infect. Dis. 2007, 13, 380–7. 10.3201/eid1303.061400. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

