Insulin receptor alternative splicing in breast and prostate cancer
- PMID: 38331804
- PMCID: PMC10851471
- DOI: 10.1186/s12935-024-03252-1
Insulin receptor alternative splicing in breast and prostate cancer
Abstract
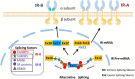
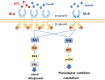
Cancer etiology represents an intricate, multifactorial orchestration where metabolically associated insulin-like growth factors (IGFs) and insulin foster cellular proliferation and growth throughout tumorigenesis. The insulin receptor (IR) exhibits two splice variants arising from alternative mRNA processing, namely IR-A, and IR-B, with remarkable distribution and biological effects disparities. This insightful review elucidates the structural intricacies, widespread distribution, and functional significance of IR-A and IR-B. Additionally, it explores the regulatory mechanisms governing alternative splicing processes, intricate signal transduction pathways, and the intricate association linking IR-A and IR-B splicing variants to breast and prostate cancer tumorigenesis. Breast cancer and prostate cancer are the most common malignant tumors with the highest incidence rates among women and men, respectively. These findings provide a promising theoretical framework for advancing preventive strategies, diagnostic modalities, and therapeutic interventions targeting breast and prostate cancer.
Keywords: Alternative splicing; Cancer; Insulin receptor isoforms.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Insulin Receptor Isoform Variations in Prostate Cancer Cells.Front Endocrinol (Lausanne). 2016 Sep 28;7:132. doi: 10.3389/fendo.2016.00132. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 27733843 Free PMC article.
-
Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease.Endocr Rev. 2009 Oct;30(6):586-623. doi: 10.1210/er.2008-0047. Epub 2009 Sep 14. Endocr Rev. 2009. PMID: 19752219 Review.
-
The Emerging Role of the Fetal Insulin Receptor in Hormone-refractory Breast Cancer.Endocrinology. 2021 Oct 1;162(10):bqab147. doi: 10.1210/endocr/bqab147. Endocrinology. 2021. PMID: 34304271 Free PMC article. Review.
-
Mitogenic insulin receptor-A is overexpressed in human hepatocellular carcinoma due to EGFR-mediated dysregulation of RNA splicing factors.Cancer Res. 2013 Jul 1;73(13):3974-86. doi: 10.1158/0008-5472.CAN-12-3824. Epub 2013 Apr 30. Cancer Res. 2013. PMID: 23633480
-
Insulin receptor (IR) and insulin-like growth factor receptor 1 (IGF-1R) signaling systems: novel treatment strategies for cancer.Med Oncol. 2014 Jan;31(1):805. doi: 10.1007/s12032-013-0805-3. Epub 2013 Dec 14. Med Oncol. 2014. PMID: 24338270 Review.
Cited by
-
Neoantigens in cancer immunotherapy: focusing on alternative splicing.Front Immunol. 2024 Jul 11;15:1437774. doi: 10.3389/fimmu.2024.1437774. eCollection 2024. Front Immunol. 2024. PMID: 39055714 Free PMC article. Review.
-
Decoding the Role of Insulin-like Growth Factor 1 and Its Isoforms in Breast Cancer.Int J Mol Sci. 2024 Aug 27;25(17):9302. doi: 10.3390/ijms25179302. Int J Mol Sci. 2024. PMID: 39273251 Free PMC article. Review.
-
Unraveling the Role of Cathepsin B Variants in Polycystic Ovary Syndrome: Insights from a Case-Control Study and Computational Analyses.Reprod Sci. 2025 Apr;32(4):1166-1179. doi: 10.1007/s43032-025-01806-w. Epub 2025 Mar 5. Reprod Sci. 2025. PMID: 40044993
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

