Macrophage subpopulations in pediatric patients with lupus nephritis and other inflammatory diseases affecting the kidney
- PMID: 38331818
- PMCID: PMC10851514
- DOI: 10.1186/s13075-024-03281-1
Macrophage subpopulations in pediatric patients with lupus nephritis and other inflammatory diseases affecting the kidney
Abstract
Background: Macrophages play an important role in the pathogenesis of lupus nephritis (LN), but less is known about macrophage subtypes in pediatric LN. Here we compared renal inflammation in LN with other inflammatory pediatric kidney diseases and assessed whether inflammation correlates with clinical parameters.
Methods: Using immunofluorescence microscopy, we analyzed renal biopsies from 20 pediatric patients with lupus nephritis (ISN/RPS classes II-V) and pediatric controls with other inflammatory kidney diseases for infiltration with M1-like (CD68 + /CD206 - , CD68 + /CD163 -), M2a-like (CD206 + /CD68 +), and M2c-like macrophages (CD163 + /CD68 +) as well as CD3 + T-cells, CD20 + B-cells, and MPO + neutrophilic granulocytes. In addition, the correlation of macrophage infiltration with clinical parameters at the time of renal biopsy, e.g., eGFR and serum urea, was investigated. Macrophage subpopulations were compared with data from a former study of adult LN patients.
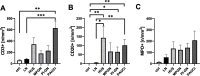
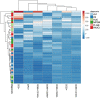
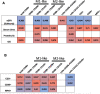
Results: The frequency of different macrophage subtypes in biopsies of pediatric LN was dependent on ISN/RPS class and showed the most pronounced M1-like macrophage infiltration in patients with LN class IV, whereas M2c-like macrophages were most abundant in class III and IV. Interestingly, on average, only half as many macrophages were found in renal biopsies of pediatric LN compared to adult patients with LN. The distribution of frequencies of macrophage subpopulations, however, was different for CD68 + CD206 + (M2a-like) but comparable for CD68 + CD163 - (M1-like) CD68 + CD163 + (M2c-like) cells in pediatric and adult patients. Compared to other inflammatory kidney diseases in children, fewer macrophages and other inflammatory cells were found in kidney biopsies of LN. Depending on the disease, the frequency of individual immune cell types varied, but we were unable to confirm disease-specific inflammatory signatures in our study due to the small number of pediatric cases. Worsened renal function, measured as elevated serum urea and decreased eGFR, correlated particularly strongly with the number of CD68 + /CD163 - M1-like macrophages and CD20 + B cells in pediatric inflammatory kidney disease.
Conclusion: Although M1-like macrophages play a greater role in pediatric LN patients than in adult LN patients, M2-like macrophages appear to be key players and are more abundant in other pediatric inflammatory kidney diseases compared to LN.
Keywords: Inflammatory kidney diseases; Lupus nephritis; Macrophage subtypes; Macrophages; Pediatric patients.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
CD163+ M2c-like macrophages predominate in renal biopsies from patients with lupus nephritis.Arthritis Res Ther. 2016 Apr 18;18:90. doi: 10.1186/s13075-016-0989-y. Arthritis Res Ther. 2016. PMID: 27091114 Free PMC article.
-
M1 and M2 Macrophage Polarization Correlates with Activity and Chronicity Indices in Lupus Nephritis.Life (Basel). 2025 Jan 4;15(1):55. doi: 10.3390/life15010055. Life (Basel). 2025. PMID: 39859995 Free PMC article.
-
Urinary soluble CD163 level reflects glomerular inflammation in human lupus nephritis.Nephrol Dial Transplant. 2016 Dec;31(12):2023-2033. doi: 10.1093/ndt/gfw214. Epub 2016 May 30. Nephrol Dial Transplant. 2016. PMID: 27242373
-
Regulation of macrophage polarization by targeted metabolic reprogramming for the treatment of lupus nephritis.Mol Med. 2024 Jun 25;30(1):96. doi: 10.1186/s10020-024-00866-z. Mol Med. 2024. PMID: 38914953 Free PMC article. Review.
-
The ISN/RPS 2003 classification of lupus nephritis: an assessment at 3 years.Kidney Int. 2007 Mar;71(6):491-5. doi: 10.1038/sj.ki.5002118. Epub 2007 Jan 31. Kidney Int. 2007. PMID: 17264872 Review.
Cited by
-
Macrophage Infiltration Correlated with IFI16, EGR1 and MX1 Expression in Renal Tubular Epithelial Cells Within Lupus Nephritis-Associated Tubulointerstitial Injury via Bioinformatics Analysis.J Inflamm Res. 2024 Dec 24;17:11469-11483. doi: 10.2147/JIR.S489087. eCollection 2024. J Inflamm Res. 2024. PMID: 39735896 Free PMC article.
References
-
- Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, D'Agati VD, Ferrario F, Haas M, Jennette JC, et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018;93(4):789–796. doi: 10.1016/j.kint.2017.11.023. - DOI - PubMed
-
- Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, Doménech I, Aydintug AO, Jedryka-Góral A, de Ramón E, et al. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. The European Working Party on Systemic Lupus Erythematosus. Medicine (Baltimore) 1993;72(2):113–124. doi: 10.1097/00005792-199303000-00005. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

