Impact of an integrated health, nutrition, and early child stimulation and responsive care intervention package delivered to preterm or term small for gestational age babies during infancy on growth and neurodevelopment: study protocol of an individually randomized controlled trial in India (Small Babies Trial)
- PMID: 38331842
- PMCID: PMC10854034
- DOI: 10.1186/s13063-024-07942-z
Impact of an integrated health, nutrition, and early child stimulation and responsive care intervention package delivered to preterm or term small for gestational age babies during infancy on growth and neurodevelopment: study protocol of an individually randomized controlled trial in India (Small Babies Trial)
Abstract
Background: Preterm and term small for gestational age (SGA) babies are at high risk of experiencing malnutrition and impaired neurodevelopment. Standalone interventions have modest and sometimes inconsistent effects on growth and neurodevelopment in these babies. For greater impact, intervention may be needed in multiple domains-health, nutrition, and psychosocial care and support. Therefore, the combined effects of an integrated intervention package for preterm and term SGA on growth and neurodevelopment are worth investigating.
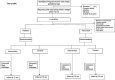
Methods: An individually randomized controlled trial is being conducted in urban and peri-urban low to middle-socioeconomic neighborhoods in South Delhi, India. Infants are randomized (1:1) into two strata of 1300 preterm and 1300 term SGA infants each to receive the intervention package or routine care. Infants will be followed until 12 months of age. Outcome data will be collected by an independent outcome ascertainment team at infant ages 1, 3, 6, 9, and 12 months and at 2, 6, and 12 months after delivery for mothers.
Discussion: The findings of this study will indicate whether providing an intervention that addresses factors known to limit growth and neurodevelopment can offer substantial benefits to preterm or term SGA infants. The results from this study will increase our understanding of growth and development and guide the design of public health programs in low- and middle-income settings for vulnerable infants.
Trial registration: The trial has been registered prospectively in Clinical Trial Registry - India # CTRI/2021/11/037881, Registered on 08 November 2021.
Keywords: Child health; Early child stimulation; Growth failure; Intrauterine growth restriction; Responsive stimulation; Small for gestational age; Small vulnerable newborns; preterm.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Similar articles
-
Impact of an integrated nutrition, health, water sanitation and hygiene, psychosocial care and support intervention package delivered during the pre- and peri-conception period and/or during pregnancy and early childhood on linear growth of infants in the first two years of life, birth outcomes and nutritional status of mothers: study protocol of a factorial, individually randomized controlled trial in India.Trials. 2020 Jan 31;21(1):127. doi: 10.1186/s13063-020-4059-z. Trials. 2020. PMID: 32005294 Free PMC article.
-
Breastfeeding practices based on the gestational age and weight at birth in the first six months of life in a population-based cohort of infants from North India.Front Pediatr. 2023 Jun 26;11:1127885. doi: 10.3389/fped.2023.1127885. eCollection 2023. Front Pediatr. 2023. PMID: 37435164 Free PMC article.
-
National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010.Lancet Glob Health. 2013 Jul;1(1):e26-36. doi: 10.1016/S2214-109X(13)70006-8. Epub 2013 Jun 25. Lancet Glob Health. 2013. PMID: 25103583 Free PMC article.
-
Cue-based versus scheduled feeding for preterm infants transitioning from tube to oral feeding: the Cubs mixed-methods feasibility study.Health Technol Assess. 2021 Dec;25(74):1-146. doi: 10.3310/hta25740. Health Technol Assess. 2021. PMID: 34878383
-
Comparison of different protein concentrations of human milk fortifier for promoting growth and neurological development in preterm infants.Cochrane Database Syst Rev. 2020 Nov 20;11(11):CD007090. doi: 10.1002/14651858.CD007090.pub2. Cochrane Database Syst Rev. 2020. PMID: 33215474 Free PMC article.
References
-
- Lee AC, Kozuki N, Cousens S, Stevens GA, Blencowe H, Silveira MF, Sania A, Rosen HE, Schmiegelow C, Adair LS, et al. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21st standard: analysis of CHERG datasets. BMJ. 2017;358:j3677. doi: 10.1136/bmj.j3677. - DOI - PMC - PubMed
-
- Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, Landoulsi S, Jampathong N, Kongwattanakul K, Laopaiboon M, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37–e46. doi: 10.1016/S2214-109X(18)30451-0. - DOI - PMC - PubMed
-
- Lawn JE, Ohuma EO, Bradley E, Idueta LS, Hazel E, Okwaraji YB, Erchick DJ, Yargawa J, Katz J, Lee ACC, et al. Small babies, big risks: global estimates of prevalence and mortality for vulnerable newborns to accelerate change and improve counting. Lancet. 2023;401(10389):1707–1719. doi: 10.1016/S0140-6736(23)00522-6. - DOI - PubMed
-
- Taneja S, Chowdhury R, Dhabhai N, Upadhyay RP, Mazumder S, Sharma S, Bhatia K, Chellani H, Dewan R, Mittal P, et al. Impact of a package of health, nutrition, psychosocial support, and WaSH interventions delivered during preconception, pregnancy, and early childhood periods on birth outcomes and on linear growth at 24 months of age: factorial, individually randomised controlled trial. BMJ. 2022;379:e072046. doi: 10.1136/bmj-2022-072046. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical