IL-15-secreting CAR natural killer cells directed toward the pan-cancer target CD70 eliminate both cancer cells and cancer-associated fibroblasts
- PMID: 38331849
- PMCID: PMC10854128
- DOI: 10.1186/s13045-024-01525-w
IL-15-secreting CAR natural killer cells directed toward the pan-cancer target CD70 eliminate both cancer cells and cancer-associated fibroblasts
Abstract
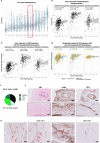
Background: It remains challenging to obtain positive outcomes with chimeric antigen receptor (CAR)-engineered cell therapies in solid malignancies, like colorectal cancer (CRC) and pancreatic ductal adenocarcinoma (PDAC). A major obstacle is the lack of targetable surface antigens that are not shared by healthy tissues. CD70 emerges as interesting target, due to its stringent expression pattern in healthy tissue and its apparent role in tumor progression in a considerable amount of malignancies. Moreover, CD70 is also expressed on cancer-associated fibroblasts (CAFs), another roadblock for treatment efficacy in CRC and PDAC. We explored the therapeutic potential of CD70 as target for CAR natural killer (NK) cell therapy in CRC, PDAC, focusing on tumor cells and CAFs, and lymphoma.
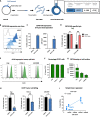
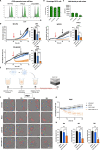
Methods: RNA-seq data and immunohistochemical analysis of patient samples were used to explore CD70 expression in CRC and PDAC patients. In addition, CD70-targeting CAR NK cells were developed to assess cytotoxic activity against CD70+ tumor cells and CAFs, and the effect of cytokine stimulation on their efficacy was evaluated. The in vitro functionality of CD70-CAR NK cells was investigated against a panel of tumor and CAF cell lines with varying CD70 expression. Lymphoma-bearing mice were used to validate in vivo potency of CD70-CAR NK cells. Lastly, to consider patient variability, CD70-CAR NK cells were tested on patient-derived organoids containing CAFs.
Results: In this study, we identified CD70 as a target for tumor cells and CAFs in CRC and PDAC patients. Functional evaluation of CD70-directed CAR NK cells indicated that IL-15 stimulation is essential to obtain effective elimination of CD70+ tumor cells and CAFs, and to improve tumor burden and survival of mice bearing CD70+ tumors. Mechanistically, IL-15 stimulation resulted in improved potency of CD70-CAR NK cells by upregulating CAR expression and increasing secretion of pro-inflammatory cytokines, in a mainly autocrine or intracellular manner.
Conclusions: We disclose CD70 as an attractive target both in hematological and solid tumors. IL-15 armored CAR NK cells act as potent effectors to eliminate these CD70+ cells. They can target both tumor cells and CAFs in patients with CRC and PDAC, and potentially other desmoplastic solid tumors.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Flieswasser T, Camara-Clayette V, Danu A, Bosq J, Ribrag V, Zabrocki P, et al. Screening a broad range of solid and haematological tumour types for CD70 expression using a uniform IHC methodology as potential patient stratification method. Cancers (Basel). 2019;11(10):1611. doi: 10.3390/cancers11101611. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

