The acidic intrinsically disordered region of the inflammatory mediator HMGB1 mediates fuzzy interactions with CXCL12
- PMID: 38331917
- PMCID: PMC10853541
- DOI: 10.1038/s41467-024-45505-7
The acidic intrinsically disordered region of the inflammatory mediator HMGB1 mediates fuzzy interactions with CXCL12
Abstract
Chemokine heterodimers activate or dampen their cognate receptors during inflammation. The CXCL12 chemokine forms with the fully reduced (fr) alarmin HMGB1 a physiologically relevant heterocomplex (frHMGB1•CXCL12) that synergically promotes the inflammatory response elicited by the G-protein coupled receptor CXCR4. The molecular details of complex formation were still elusive. Here we show by an integrated structural approach that frHMGB1•CXCL12 is a fuzzy heterocomplex. Unlike previous assumptions, frHMGB1 and CXCL12 form a dynamic equimolar assembly, with structured and unstructured frHMGB1 regions recognizing the CXCL12 dimerization surface. We uncover an unexpected role of the acidic intrinsically disordered region (IDR) of HMGB1 in heterocomplex formation and its binding to CXCR4 on the cell surface. Our work shows that the interaction of frHMGB1 with CXCL12 diverges from the classical rigid heterophilic chemokines dimerization. Simultaneous interference with multiple interactions within frHMGB1•CXCL12 might offer pharmacological strategies against inflammatory conditions.
© 2024. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: L.S.C. is an employee and M.E.B. is founder and part-owner of HMGBiotech, a company that provides goods and services related to HMGB proteins. The remaining authors declare no competing interests.
Figures

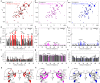

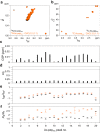
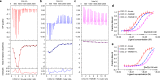
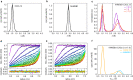


References
MeSH terms
Substances
Grants and funding
- IG 2018 - ID 21440/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- 24118/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- MFAG27415/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)
- IG 2020 - ID. 24702/Associazione Italiana per la Ricerca sul Cancro (Italian Association for Cancer Research)

