Major depressive disorder: hypothesis, mechanism, prevention and treatment
- PMID: 38331979
- PMCID: PMC10853571
- DOI: 10.1038/s41392-024-01738-y
Major depressive disorder: hypothesis, mechanism, prevention and treatment
Abstract
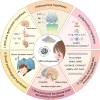
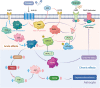
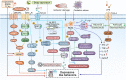
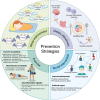
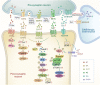
Worldwide, the incidence of major depressive disorder (MDD) is increasing annually, resulting in greater economic and social burdens. Moreover, the pathological mechanisms of MDD and the mechanisms underlying the effects of pharmacological treatments for MDD are complex and unclear, and additional diagnostic and therapeutic strategies for MDD still are needed. The currently widely accepted theories of MDD pathogenesis include the neurotransmitter and receptor hypothesis, hypothalamic-pituitary-adrenal (HPA) axis hypothesis, cytokine hypothesis, neuroplasticity hypothesis and systemic influence hypothesis, but these hypothesis cannot completely explain the pathological mechanism of MDD. Even it is still hard to adopt only one hypothesis to completely reveal the pathogenesis of MDD, thus in recent years, great progress has been made in elucidating the roles of multiple organ interactions in the pathogenesis MDD and identifying novel therapeutic approaches and multitarget modulatory strategies, further revealing the disease features of MDD. Furthermore, some newly discovered potential pharmacological targets and newly studied antidepressants have attracted widespread attention, some reagents have even been approved for clinical treatment and some novel therapeutic methods such as phototherapy and acupuncture have been discovered to have effective improvement for the depressive symptoms. In this work, we comprehensively summarize the latest research on the pathogenesis and diagnosis of MDD, preventive approaches and therapeutic medicines, as well as the related clinical trials.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

