Dolutegravir based therapy showed CD4+ T cell count recovery and viral load suppression among ART naïve people living with HIV AIDS: a pilot evaluation
- PMID: 38331983
- PMCID: PMC10853173
- DOI: 10.1038/s41598-024-53282-y
Dolutegravir based therapy showed CD4+ T cell count recovery and viral load suppression among ART naïve people living with HIV AIDS: a pilot evaluation
Abstract
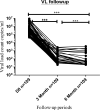
Recently, dolutegravir (DTG)-based combined therapy, a more effective and safer first-line antiretroviral therapy (ART), has been recommended by the World Health Organization for the treatment of Human Immunodeficiency Virus (HIV) since July 2018. However, its effectiveness in CD4+ T-cells count recovery and viral load suppression has not been studied yet in Ethiopia, where HIV is endemic. Therefore, we aimed to conduct a pilot assessment on the effect of DTG-based therapy on CD4+ T-cell count and viral load count among people living with HIV (PLWH) in Ethiopia. A longitudinal prospective cohort study was conducted from July 2020 to February 2021. 109 PLWH who are ART naive but plan to initiate DTG-based therapy were recruited. HIV viral ribonucleic acid (RNA) copies were determined using polymerase chain reaction. To compute the difference in viral load and CD4+ T-cell counts between the baseline, 3rd, and 6th months, a Friedman test was used. The study included 109 PLWH who had never received antiretroviral medication. Participants taking DTG-based treatment showed significantly decreasing median (IQR) values of viral load count (copies/mL) from 446,812 (237649.5-732994.5) at baseline to 34 (23.5-46) at 3 months and 0.0 (0-19) at 6 months of treatment follow-up. Although the treatment increases the proportion of participants with HIV-1 RNA 50 copies/mL from 0 (0% at baseline) to 87 (79.8%) and 100 (91.7%) at the 3rd and 6th months of treatment, respectively, On the other hand, the CD4+ T-cell count increased significantly during treatment: median (IQR): 209 (81.5-417.5) versus 291 (132-522) versus 378 (181-632.5) cells/L at baseline, the 3rd and 6th months of the treatment follow-up period, respectively. We found dolutegravir-based therapy was a promising option with high virological suppression rates and CD4+ T-cell count recovery, demonstrating a restoration of cellular immunity. Moreover, Viral load suppression rates were high after the initiation of the treatment. We recommend further research should be conducted with a larger number of participants to acquire greater awareness of the treatment outcomes.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- WHO. Clinical Guidelines: Antiretroviral Therapy. Consol Guidel Use Antiretrovir Drugs Treat Prev HIV Infect Recomm a Public Heal Approach (Second Edition):129; http://www.who.int/hiv/pub/arv/chapter4.pdf?ua=1 (2016).
-
- Llibre JM, Cahn PE, Lo J, Barber TJ, Mussini C, van Welzen BJ, et al. Changes in inflammatory and atherogenesis biomarkers with the 2-drug regimen dolutegravir plus lamivudine in antiretroviral therapy-experienced, virologically suppressed people with HIV-1: A systematic literature review. Open Forum Infect. Dis. 2022;9(4):2–10. doi: 10.1093/ofid/ofac068. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

