tRNA epitranscriptomic alterations associated with opioid-induced reward-seeking and long-term opioid withdrawal in male mice
- PMID: 38332016
- PMCID: PMC11224224
- DOI: 10.1038/s41386-024-01813-6
tRNA epitranscriptomic alterations associated with opioid-induced reward-seeking and long-term opioid withdrawal in male mice
Abstract
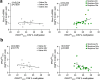
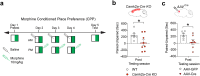
DNA cytosine methylation has been documented as a potential epigenetic mechanism of transcriptional regulation underlying opioid use disorder. However, methylation of RNA cytosine residues, which would drive another level of biological influence as an epitranscriptomic mechanism of gene and protein regulation has not been studied in the context of addiction. Here, we probed whether chronic morphine exposure could alter tRNA cytosine methylation (m5C) and resulting expression levels in the medial prefrontal cortex (mPFC), a brain region crucial for reward processing and executive function that exhibits opioid-induced molecular restructuring. We identified dynamic changes in glycine tRNA (tRNAGlyGCC) cytosine methylation, corresponding to altered expression levels of this tRNA at multiple timepoints following 15 days of daily morphine. Additionally, a robust increase in methylation, coupled with decreased expression, was present after 30 days of withdrawal, suggesting that repeated opioid administration produces changes to the tRNA regulome long after discontinuation. Furthermore, forebrain-wide knockout of neuronal Nsun2, a tRNA methyltransferase, was associated with disruption of opioid conditioned place preference, and this effect was recapitulated by regional mPFC Nsun2 knockout. Taken together, these studies provide a foundational link between the regulation of tRNA cytosine methylation and opioid reward and highlight the tRNA machinery as a potential therapeutic target in addiction.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Neuronal Nsun2 deficiency produces tRNA epitranscriptomic alterations and proteomic shifts impacting synaptic signaling and behavior.Nat Commun. 2021 Aug 13;12(1):4913. doi: 10.1038/s41467-021-24969-x. Nat Commun. 2021. PMID: 34389722 Free PMC article.
-
The effects of AMPA receptor blockade in the prelimbic cortex on systemic and ventral tegmental area opiate reward sensitivity.Psychopharmacology (Berl). 2013 Feb;225(3):687-95. doi: 10.1007/s00213-012-2852-4. Epub 2012 Sep 13. Psychopharmacology (Berl). 2013. PMID: 22972411
-
Decreased Neuronal Excitability in Medial Prefrontal Cortex during Morphine Withdrawal is associated with enhanced SK channel activity and upregulation of small GTPase Rac1.Theranostics. 2020 Jun 5;10(16):7369-7383. doi: 10.7150/thno.44893. eCollection 2020. Theranostics. 2020. PMID: 32641997 Free PMC article.
-
A medial prefrontal cortex-nucleus acumens corticotropin-releasing factor circuitry for neuropathic pain-increased susceptibility to opioid reward.Transl Psychiatry. 2018 May 21;8(1):100. doi: 10.1038/s41398-018-0152-4. Transl Psychiatry. 2018. PMID: 29780165 Free PMC article.
-
Drug-sensitive reward in crayfish: an invertebrate model system for the study of SEEKING, reward, addiction, and withdrawal.Neurosci Biobehav Rev. 2011 Oct;35(9):1847-53. doi: 10.1016/j.neubiorev.2010.12.008. Epub 2010 Dec 21. Neurosci Biobehav Rev. 2011. PMID: 21182861 Free PMC article. Review.
Cited by
-
Altered tRNA expression profile associated with codon-specific proteomic changes in the suicide brain.Mol Psychiatry. 2025 Jul;30(7):2871-2879. doi: 10.1038/s41380-025-02891-8. Epub 2025 Jan 14. Mol Psychiatry. 2025. PMID: 39809846 Free PMC article.
-
Neuron-Specific Glycine Metabolism Links Transfer RNA Epitranscriptomic Regulation to Complex Behaviors.Biol Psychiatry Glob Open Sci. 2024 Dec 14;5(2):100432. doi: 10.1016/j.bpsgos.2024.100432. eCollection 2025 Mar. Biol Psychiatry Glob Open Sci. 2024. PMID: 39911537 Free PMC article.
-
An Investigation of the RNA Modification m6A and Its Regulatory Enzymes in Rat Brains Affected by Chronic Morphine Treatment and Withdrawal.Int J Mol Sci. 2025 May 4;26(9):4371. doi: 10.3390/ijms26094371. Int J Mol Sci. 2025. PMID: 40362608 Free PMC article.
References
-
- Results from the 2021 National Survey on Drug Use and Health (NSDUH): Key Substance Use and Mental Health Indicators in the United States. Rockville, MD: SAMHSA Publications; 2023.
-
- Ahmad FB, Cisewski JA, Rossen LM, Sutton P. Provisional drug overdose death counts. National Center for Health Statistics. 2023.
MeSH terms
Substances
Grants and funding
- P01 DA047233/DA/NIDA NIH HHS/United States
- R01 DA007359/DA/NIDA NIH HHS/United States
- R01 DA054526/DA/NIDA NIH HHS/United States
- R01 NS111351/NS/NINDS NIH HHS/United States
- R01 NS086444/NS/NINDS NIH HHS/United States
- R01NS086444/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01DA054526/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)
- P01DA047233/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)
- R01DA007359/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)
LinkOut - more resources
Full Text Sources
Miscellaneous

