Trophoblast stem cell-based organoid models of the human placental barrier
- PMID: 38332125
- PMCID: PMC10853531
- DOI: 10.1038/s41467-024-45279-y
Trophoblast stem cell-based organoid models of the human placental barrier
Abstract
Human placental villi have essential roles in producing hormones, mediating nutrient and waste exchange, and protecting the fetus from exposure to xenobiotics. Human trophoblast organoids that recapitulate the structure of villi could provide an important in vitro tool to understand placental development and the transplacental passage of xenobiotics. However, such organoids do not currently exist. Here we describe the generation of trophoblast organoids using human trophoblast stem (TS) cells. Following treatment with three kinds of culture medium, TS cells form spherical organoids with a single outer layer of syncytiotrophoblast (ST) cells that display a barrier function. Furthermore, we develop a column-type ST barrier model based on the culture condition of the trophoblast organoids. The bottom membrane of the column is almost entirely covered with syndecan 1-positive ST cells. The barrier integrity and maturation levels of the model are confirmed by measuring transepithelial/transendothelial electrical resistance (TEER) and the amount of human chorionic gonadotropin. Further analysis reveals that the model can be used to derive the apparent permeability coefficients of model compounds. In addition to providing a suite of tools for the study of placental development, our trophoblast models allow the evaluation of compound transfer and toxicity, which will facilitate drug development.
© 2024. The Author(s).
Conflict of interest statement
TMDU has filed a patent application covering the protocol and methods for the generation of human trophoblast organoids. T.H. and H.K. own stock as members of a recently established company, HPS Inc., and they potentially would receive compensation from the company. The remaining authors declare no competing interests.
Figures
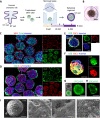
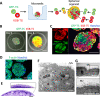

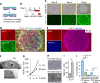
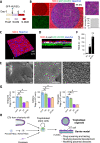
Similar articles
-
Trophoblast organoids as a model for maternal-fetal interactions during human placentation.Nature. 2018 Dec;564(7735):263-267. doi: 10.1038/s41586-018-0753-3. Epub 2018 Nov 28. Nature. 2018. PMID: 30487605 Free PMC article.
-
Trophoblast Organoids as a Novel Tool to Study Human Placental Development and Function.Methods Mol Biol. 2024;2728:195-222. doi: 10.1007/978-1-0716-3495-0_17. Methods Mol Biol. 2024. PMID: 38019403
-
Stem-cell-derived trophoblast organoids model human placental development and susceptibility to emerging pathogens.Cell Stem Cell. 2022 May 5;29(5):810-825.e8. doi: 10.1016/j.stem.2022.04.004. Cell Stem Cell. 2022. PMID: 35523141 Free PMC article.
-
Progress in deciphering trophoblast cell differentiation during human placentation.Curr Opin Cell Biol. 2020 Dec;67:86-91. doi: 10.1016/j.ceb.2020.08.010. Epub 2020 Sep 18. Curr Opin Cell Biol. 2020. PMID: 32957014 Review.
-
Trophoblast stem cells, trophoblast invasion, and organoids - advancements in gynecology.Ceska Gynekol. 2024;89(2):151-155. doi: 10.48095/cccg2024151. Ceska Gynekol. 2024. PMID: 38704229 Review. English.
Cited by
-
Single-cell RNA sequencing reveals placental response under environmental stress.Nat Commun. 2024 Aug 2;15(1):6549. doi: 10.1038/s41467-024-50914-9. Nat Commun. 2024. PMID: 39095385 Free PMC article.
-
Epigenetic dynamics of partially methylated domains in human placenta and trophoblast stem cells.BMC Genomics. 2024 Nov 6;25(1):1050. doi: 10.1186/s12864-024-10986-9. BMC Genomics. 2024. PMID: 39506688 Free PMC article.
-
Hypoxia and loss of GCM1 expression prevent differentiation and contact inhibition in human trophoblast stem cells.Stem Cell Reports. 2025 May 13;20(5):102481. doi: 10.1016/j.stemcr.2025.102481. Epub 2025 Apr 24. Stem Cell Reports. 2025. PMID: 40280139 Free PMC article.
-
Current strategies using 3D organoids to establish in vitro maternal-embryonic interaction.J Vet Sci. 2024 May;25(3):e40. doi: 10.4142/jvs.24004. J Vet Sci. 2024. PMID: 38834510 Free PMC article. Review.
-
Organoids in Haematologic Research: Advances and Future Directions.Cell Prolif. 2025 Jun;58(6):e13806. doi: 10.1111/cpr.13806. Epub 2025 Jan 26. Cell Prolif. 2025. PMID: 40538372 Free PMC article. Review.
References
MeSH terms
Grants and funding
- JP21gm1310001/Japan Agency for Medical Research and Development (AMED)
- 22K18936/MEXT | Japan Society for the Promotion of Science (JSPS)
- 21K04852/MEXT | Japan Society for the Promotion of Science (JSPS)
- JPMJTM22BD/MEXT | JST | Strategic Promotion of Innovative R and D (Strategic Promotion of Innovative R&D)
LinkOut - more resources
Full Text Sources
Research Materials

