Virological characteristics of the SARS-CoV-2 Omicron XBB.1.5 variant
- PMID: 38332154
- PMCID: PMC10853506
- DOI: 10.1038/s41467-024-45274-3
Virological characteristics of the SARS-CoV-2 Omicron XBB.1.5 variant
Abstract
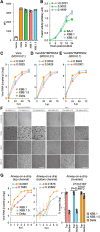
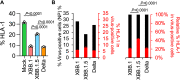
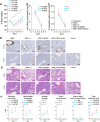
Circulation of SARS-CoV-2 Omicron XBB has resulted in the emergence of XBB.1.5, a new Variant of Interest. Our phylogenetic analysis suggests that XBB.1.5 evolved from XBB.1 by acquiring the S486P spike (S) mutation, subsequent to the acquisition of a nonsense mutation in ORF8. Neutralization assays showed similar abilities of immune escape between XBB.1.5 and XBB.1. We determine the structural basis for the interaction between human ACE2 and the S protein of XBB.1.5, showing similar overall structures between the S proteins of XBB.1 and XBB.1.5. We provide the intrinsic pathogenicity of XBB.1 and XBB.1.5 in hamsters. Importantly, we find that the ORF8 nonsense mutation of XBB.1.5 resulted in impairment of MHC suppression. In vivo experiments using recombinant viruses reveal that the XBB.1.5 mutations are involved with reduced virulence of XBB.1.5. Together, our study identifies the two viral functions defined the difference between XBB.1 and XBB.1.5.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
- JP21nf0101627/Japan Agency for Medical Research and Development (AMED)
- JP21fk0108493/Japan Agency for Medical Research and Development (AMED)
- JP22fk0108617/Japan Agency for Medical Research and Development (AMED)
- JP22fk0108516/Japan Agency for Medical Research and Development (AMED)
- JP22gm1610008/Japan Agency for Medical Research and Development (AMED)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

