Beyond the 510(k): The regulation of novel moderate-risk medical devices, intellectual property considerations, and innovation incentives in the FDA's De Novo pathway
- PMID: 38332182
- PMCID: PMC10853500
- DOI: 10.1038/s41746-024-01021-y
Beyond the 510(k): The regulation of novel moderate-risk medical devices, intellectual property considerations, and innovation incentives in the FDA's De Novo pathway
Abstract
Moderate-risk medical devices constitute 99% of those that have been regulated by the U.S. Food and Drug Administration (FDA) since it gained authority to regulate medical technology nearly five decades ago. This article presents an analysis of the interaction between the 510(k) process -the historically dominant path to market for most medical devices- and the De Novo pathway, a more recent alternative that targets more novel devices, including those involving new technologies, diagnostics, hardware, and software. The De Novo pathway holds significant potential for innovators seeking to define new categories of medical devices, as it represents a less burdensome approach than would have otherwise been needed historically. Moreover, it supports the FDA in its effort to modernize the long-established 510(k) pathway by promoting the availability of up-to-date device "predicates" upon which subsequent device applications can be based, reflecting positive spillovers that are likely to encourage manufacturers to adopt current state-of-the-art technologies and modern standards of safety and effectiveness. We analyze the of characteristics all the De Novo classification requests to date, including the submission type, trends, FDA review times, and device types. After characterizing how the De Novo process has been used over time, we discuss its unique challenges and opportunities with respect to medical device software and AI-enabled devices, including considerations for intellectual property, innovation, and competition economics.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
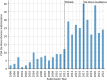
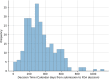
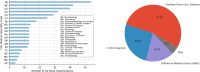
References
-
- Medical Device Market. Facts & Factors, Vol. 282 (Medical Device Market, 2021).
-
- FDA. Premarket Notification 510(k). https://www.fda.gov/medical-devices/premarket-submissions-selecting-and-... (2022).
-
- FDA. Device Classification Panels. https://www.fda.gov/medical-devices/classify-your-medical-device/device-... (2018).
Grants and funding
LinkOut - more resources
Full Text Sources

