40 Hz light flickering promotes sleep through cortical adenosine signaling
- PMID: 38332199
- PMCID: PMC10907382
- DOI: 10.1038/s41422-023-00920-1
40 Hz light flickering promotes sleep through cortical adenosine signaling
Abstract
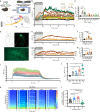
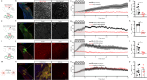
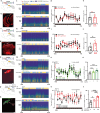
Flickering light stimulation has emerged as a promising non-invasive neuromodulation strategy to alleviate neuropsychiatric disorders. However, the lack of a neurochemical underpinning has hampered its therapeutic development. Here, we demonstrate that light flickering triggered an immediate and sustained increase (up to 3 h after flickering) in extracellular adenosine levels in the primary visual cortex (V1) and other brain regions, as a function of light frequency and intensity, with maximal effects observed at 40 Hz frequency and 4000 lux. We uncovered cortical (glutamatergic and GABAergic) neurons, rather than astrocytes, as the cellular source, the intracellular adenosine generation from AMPK-associated energy metabolism pathways (but not SAM-transmethylation or salvage purine pathways), and adenosine efflux mediated by equilibrative nucleoside transporter-2 (ENT2) as the molecular pathway responsible for extracellular adenosine generation. Importantly, 40 Hz (but not 20 and 80 Hz) light flickering for 30 min enhanced non-rapid eye movement (non-REM) and REM sleep for 2-3 h in mice. This somnogenic effect was abolished by ablation of V1 (but not superior colliculus) neurons and by genetic deletion of the gene encoding ENT2 (but not ENT1), but recaptured by chemogenetic inhibition of V1 neurons and by focal infusion of adenosine into V1 in a dose-dependent manner. Lastly, 40 Hz light flickering for 30 min also promoted sleep in children with insomnia by decreasing sleep onset latency, increasing total sleep time, and reducing waking after sleep onset. Collectively, our findings establish the ENT2-mediated adenosine signaling in V1 as the neurochemical basis for 40 Hz flickering-induced sleep and unravel a novel and non-invasive treatment for insomnia, a condition that affects 20% of the world population.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
- 82101556/National Natural Science Foundation of China (National Science Foundation of China)
- 31970948/National Natural Science Foundation of China (National Science Foundation of China)
- 31600859/National Natural Science Foundation of China (National Science Foundation of China)
- 81971064/National Natural Science Foundation of China (National Science Foundation of China)
- 81971031/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

