13C-Metabolic flux analysis detected a hyperoxemia-induced reduction of tricarboxylic acid cycle metabolism in granulocytes during two models of porcine acute subdural hematoma and hemorrhagic shock
- PMID: 38332911
- PMCID: PMC10850868
- DOI: 10.3389/fimmu.2023.1319986
13C-Metabolic flux analysis detected a hyperoxemia-induced reduction of tricarboxylic acid cycle metabolism in granulocytes during two models of porcine acute subdural hematoma and hemorrhagic shock
Abstract
Introduction: Supplementation with increased inspired oxygen fractions has been suggested to alleviate the harmful effects of tissue hypoxia during hemorrhagic shock (HS) and traumatic brain injury. However, the utility of therapeutic hyperoxia in critical care is disputed to this day as controversial evidence is available regarding its efficacy. Furthermore, in contrast to its hypoxic counterpart, the effect of hyperoxia on the metabolism of circulating immune cells remains ambiguous. Both stimulating and detrimental effects are possible; the former by providing necessary oxygen supply, the latter by generation of excessive amounts of reactive oxygen species (ROS). To uncover the potential impact of increased oxygen fractions on circulating immune cells during intensive care, we have performed a 13C-metabolic flux analysis (MFA) on PBMCs and granulocytes isolated from two long-term, resuscitated models of combined acute subdural hematoma (ASDH) and HS in pigs with and without cardiovascular comorbidity.
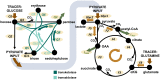
Methods: Swine underwent resuscitation after 2 h of ASDH and HS up to a maximum of 48 h after HS. Animals received normoxemia (PaO2 = 80 - 120 mmHg) or targeted hyperoxemia (PaO2 = 200 - 250 mmHg for 24 h after treatment initiation, thereafter PaO2 as in the control group). Blood was drawn at time points T1 = after instrumentation, T2 = 24 h post ASDH and HS, and T3 = 48 h post ASDH and HS. PBMCs and granulocytes were isolated from whole blood to perform electron spin resonance spectroscopy, high resolution respirometry and 13C-MFA. For the latter, we utilized a parallel tracer approach with 1,2-13C2 glucose, U-13C glucose, and U-13C glutamine, which covered essential pathways of glucose and glutamine metabolism and supplied redundant data for robust Bayesian estimation. Gas chromatography-mass spectrometry further provided multiple fragments of metabolites which yielded additional labeling information. We obtained precise estimations of the fluxes, their joint credibility intervals, and their relations, and characterized common metabolic patterns with principal component analysis (PCA).
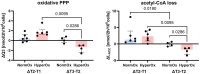
Results: 13C-MFA indicated a hyperoxia-mediated reduction in tricarboxylic acid (TCA) cycle activity in circulating granulocytes which encompassed fluxes of glutamine uptake, TCA cycle, and oxaloacetate/aspartate supply for biosynthetic processes. We further detected elevated superoxide levels in the swine strain characterized by a hypercholesterolemic phenotype. PCA revealed cell type-specific behavioral patterns of metabolic adaptation in response to ASDH and HS that acted irrespective of swine strains or treatment group.
Conclusion: In a model of resuscitated porcine ASDH and HS, we saw that ventilation with increased inspiratory O2 concentrations (PaO2 = 200 - 250 mmHg for 24 h after treatment initiation) did not impact mitochondrial respiration of PBMCs or granulocytes. However, Bayesian 13C-MFA results indicated a reduction in TCA cycle activity in granulocytes compared to cells exposed to normoxemia in the same time period. This change in metabolism did not seem to affect granulocytes' ability to perform phagocytosis or produce superoxide radicals.
Keywords: Bayesian modeling; glucose metabolism; glutamine utilization; hyperoxia; immunometabolism; mass isotopomer distribution; peripheral blood mononuclear cells; reactive oxygen species.
Copyright © 2024 Wolfschmitt, Vogt, Hogg, Wachter, Stadler, Kapapa, Datzmann, Messerer, Hoffmann, Gröger, Münz, Mathieu, Mayer, Merz, Asfar, Calzia, Radermacher and Zink.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
The effect of sodium thiosulfate on immune cell metabolism during porcine hemorrhage and resuscitation.Front Immunol. 2023 Feb 23;14:1125594. doi: 10.3389/fimmu.2023.1125594. eCollection 2023. Front Immunol. 2023. PMID: 36911662 Free PMC article.
-
The effect of targeted hyperoxemia in a randomized controlled trial employing a long-term resuscitated, model of combined acute subdural hematoma and hemorrhagic shock in swine with coronary artery disease: An exploratory, hypothesis-generating study.Front Med (Lausanne). 2022 Aug 22;9:971882. doi: 10.3389/fmed.2022.971882. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36072939 Free PMC article.
-
An exploratory study investigating the effect of targeted hyperoxemia in a randomized controlled trial in a long-term resuscitated model of combined acute subdural hematoma and hemorrhagic shock in cardiovascular healthy pigs.Front Immunol. 2023 Apr 11;14:1123196. doi: 10.3389/fimmu.2023.1123196. eCollection 2023. Front Immunol. 2023. PMID: 37114041 Free PMC article.
-
Tricarboxylic acid cycle intermediate pool size: functional importance for oxidative metabolism in exercising human skeletal muscle.Sports Med. 2007;37(12):1071-88. doi: 10.2165/00007256-200737120-00005. Sports Med. 2007. PMID: 18027994 Review.
-
Reconstruction and flux analysis of coupling between metabolic pathways of astrocytes and neurons: application to cerebral hypoxia.Theor Biol Med Model. 2007 Dec 10;4:48. doi: 10.1186/1742-4682-4-48. Theor Biol Med Model. 2007. PMID: 18070347 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

