Efficacy and activity of treatments after progression from palbociclib plus endocrine therapy in patients with HR+/HER2- metastatic breast cancer: a prospective, monocentric study
- PMID: 38332945
- PMCID: PMC10852029
- DOI: 10.7573/dic.2023-7-5
Efficacy and activity of treatments after progression from palbociclib plus endocrine therapy in patients with HR+/HER2- metastatic breast cancer: a prospective, monocentric study
Abstract
Background: Breast cancer is the most frequent tumour worldwide, and the HR+/HER2- subtype is the most common. For this tumour type, endocrine therapy (ET) is the mainstay of treatment. The association of ET and CDK4/6 inhibitors (CDK4/6i) represents the gold standard for first-line or second-line therapies. However, the optimal therapeutic strategy after CDK4/6i progression is still a matter of debate, with several randomized clinical trials still ongoing.
Patients and methods: This is an observational, prospective, real-world study including women with HR+/HER2- metastatic breast cancer progressing to palbociclib plus ET. Patients received either ET or chemotherapy (CT). The primary objective was the evaluation of efficacy of the different therapeutic strategies after palbociclib in terms of median progression-free survival 2. Secondary objectives were the activity of therapeutic strategies measured with the clinical benefit rate, evaluation of the parameters used for the treatment choice, and progression-free survival 1 related to palbociclib plus ET treatment.
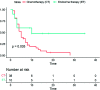
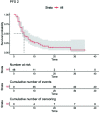
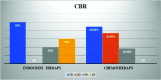
Results: Overall, 48 patients (median age 53, range 33-78 years) were included. The median progression-free survival 2 was of 5 months in the overall cohort (95% CI 4-48 months) with a statistically significant difference between the two therapeutic strategies adopted (ET versus CT, 10 months versus 5 months, respectively). Regarding secondary objectives, the clinical benefit rate was 55.2% in the CT cohort and 50% in ET. Moreover, women treated with CT had a greater number of visceral metastases and a shorter median progression-free survival 1 than patients who received ET.
Conclusions: ET and CT represent two possible therapeutic alternatives for patients progressing on CDK4/6i plus ET. The choice is based on clinical parameters, with a potential preference for ET.
Keywords: endocrine resistance; metastatic breast cancer; palbociclib; post-progression; therapeutic strategies.
Copyright © 2024 Palumbo R, Quaquarini E, Saltalamacchia G, Malovini A, Lapidari P, Tagliaferri B, Mollica L, Teragni CM, Barletta C, Locati LD, Sottotetti F.
Conflict of interest statement
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2023/11/dic.2023-7-5-COI.pdf
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

