AI algorithm for personalized resource allocation and treatment of hemorrhage casualties
- PMID: 38332989
- PMCID: PMC10851938
- DOI: 10.3389/fphys.2024.1327948
AI algorithm for personalized resource allocation and treatment of hemorrhage casualties
Abstract
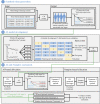
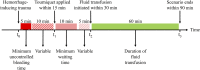
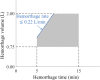
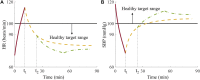
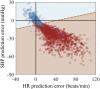
A deep neural network-based artificial intelligence (AI) model was assessed for its utility in predicting vital signs of hemorrhage patients and optimizing the management of fluid resuscitation in mass casualties. With the use of a cardio-respiratory computational model to generate synthetic data of hemorrhage casualties, an application was created where a limited data stream (the initial 10 min of vital-sign monitoring) could be used to predict the outcomes of different fluid resuscitation allocations 60 min into the future. The predicted outcomes were then used to select the optimal resuscitation allocation for various simulated mass-casualty scenarios. This allowed the assessment of the potential benefits of using an allocation method based on personalized predictions of future vital signs versus a static population-based method that only uses currently available vital-sign information. The theoretical benefits of this approach included up to 46% additional casualties restored to healthy vital signs and a 119% increase in fluid-utilization efficiency. Although the study is not immune from limitations associated with synthetic data under specific assumptions, the work demonstrated the potential for incorporating neural network-based AI technologies in hemorrhage detection and treatment. The simulated injury and treatment scenarios used delineated possible benefits and opportunities available for using AI in pre-hospital trauma care. The greatest benefit of this technology lies in its ability to provide personalized interventions that optimize clinical outcomes under resource-limited conditions, such as in civilian or military mass-casualty events, involving moderate and severe hemorrhage.
Keywords: artificial intelligence; fluid resuscitation; hemorrhage; resource utilization; trauma.
Copyright © 2024 Jin, Frock, Nagaraja, Wallqvist and Reifman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





References
-
- Alsalti M., Tivay A., Jin X., Kramer G. C., Hahn J.-O. (2022). Design and in silico evaluation of a closed-loop hemorrhage resuscitation algorithm with blood pressure as controlled variable. J. Dyn. Syst. Meas. Control. 144, 021001. 10.1115/1.4052312 - DOI
-
- Bray A., Webb J. B., Enquobahrie A., Vicory J., Heneghan J., Hubal R., et al. (2019). Pulse physiology engine: an open-source software platform for computational modeling of human medical simulation. SN Comp. Clin. Med. 1, 362–377. 10.1007/s42399-019-00053-w - DOI
-
- Burges C. J. (1998). A tutorial on support vector machines for pattern recognition. Data Min. Knowl. Discov. 2, 121–167. 10.1023/A:1009715923555 - DOI
LinkOut - more resources
Full Text Sources

