Effects of unburned tobacco smoke on inflammatory and oxidative mediators in the rat prefrontal cortex
- PMID: 38333013
- PMCID: PMC10851081
- DOI: 10.3389/fphar.2024.1328917
Effects of unburned tobacco smoke on inflammatory and oxidative mediators in the rat prefrontal cortex
Abstract
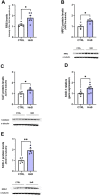
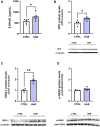
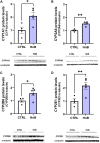
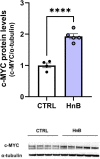
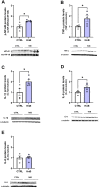
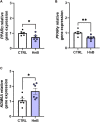
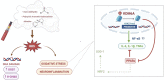
Although the Food and Drug Administration has authorized the marketing of "heat-not-burn" (HnB) electronic cigarettes as a modified risk tobacco product (MRTP), toxicological effects of HnB smoke exposure on the brain are still unexplored. Here, paramagnetic resonance of the prefrontal cortex (PFC) of HnB-exposed rats shows a dramatic increase in reactive radical species (RRS) yield coupled with an inflammatory response mediated by NF-κB-target genes including TNF-α, IL-1β, and IL-6 and the downregulation of peroxisome proliferator-activated receptor (PPAR) alpha and gamma expression. The PFC shows higher levels of 8-hydroxyguanosine, a marker of DNA oxidative damage, along with the activation of antioxidant machinery and DNA repair systems, including xeroderma pigmentosum group C (XPC) protein complex and 8-oxoguanine DNA glycosylase 1. HnB also induces the expression of drug-metabolizing enzymes such as CYP1A1, CYP2A6, CYP2B6, and CYP2E, particularly involved in the biotransformation of nicotine and several carcinogenic agents such as aldehydes and polycyclic aromatic hydrocarbons here recorded in the HnB stick smoke. Taken together, these effects, from disruption of redox homeostasis, inflammation, PPAR manipulation along with enhanced bioactivation of neurotoxicants, and upregulation of cMYC protooncogene to impairment of primary cellular defense mechanisms, suggest a possible increased risk of brain cancer. Although the HnB device reduces the emission of tobacco toxicants, our findings indicate that its consumption may carry a risk of potential adverse health effects, especially in non-smokers so far. Further studies are needed to fully understand the long-term effects of these devices.
Keywords: KDMs; e-cigarette; heat-not-burn; inflammation; oxidative stress; peroxisome proliferator-activated receptors; prefrontal cortex.
Copyright © 2024 Vivarelli, Morosini, Rullo, Losapio, Lacorte, Sangiorgi, Ghini, Fagiolino, Franchi, Lucarini, Candeletti, Canistro, Romualdi and Paolini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Heat-not-burn technology affects plasma testosterone levels and markers of inflammation, oxidative stress in the testes of rats.Front Toxicol. 2025 Jan 20;6:1515850. doi: 10.3389/ftox.2024.1515850. eCollection 2024. Front Toxicol. 2025. PMID: 39902465 Free PMC article.
-
Unburned Tobacco Cigarette Smoke Alters Rat Ultrastructural Lung Airways and DNA.Nicotine Tob Res. 2021 Nov 5;23(12):2127-2134. doi: 10.1093/ntr/ntab108. Nicotine Tob Res. 2021. PMID: 34036368
-
Heat-Not-Burn cigarette induces oxidative stress response in primary rat alveolar epithelial cells.PLoS One. 2020 Nov 25;15(11):e0242789. doi: 10.1371/journal.pone.0242789. eCollection 2020. PLoS One. 2020. PMID: 33237957 Free PMC article.
-
IQOS - a heat-not-burn (HnB) tobacco product - chemical composition and possible impact on oxidative stress and inflammatory response. A systematic review.Toxicol Mech Methods. 2020 Feb;30(2):81-87. doi: 10.1080/15376516.2019.1669245. Epub 2019 Oct 2. Toxicol Mech Methods. 2020. PMID: 31532297
-
Human Biomarker Exposure From Cigarettes Versus Novel Heat-Not-Burn Devices: A Systematic Review and Meta-Analysis.Nicotine Tob Res. 2020 Jun 12;22(7):1077-1085. doi: 10.1093/ntr/ntz200. Nicotine Tob Res. 2020. PMID: 31641752
Cited by
-
Heat-not-burn technology affects plasma testosterone levels and markers of inflammation, oxidative stress in the testes of rats.Front Toxicol. 2025 Jan 20;6:1515850. doi: 10.3389/ftox.2024.1515850. eCollection 2024. Front Toxicol. 2025. PMID: 39902465 Free PMC article.
-
Unburned Tobacco Smoke Affects Neuroinflammation-Related Pathways in the Rat Mesolimbic System.Int J Mol Sci. 2024 May 11;25(10):5259. doi: 10.3390/ijms25105259. Int J Mol Sci. 2024. PMID: 38791298 Free PMC article.
-
World no-tobacco: effects of second-hand smoke (SHS) and vapors on the developing and adult brain.Front Pharmacol. 2025 Mar 6;16:1466332. doi: 10.3389/fphar.2025.1466332. eCollection 2025. Front Pharmacol. 2025. PMID: 40115268 Free PMC article. Review.
-
Green Tea Polyphenol Nanoparticles Reduce Anxiety Caused by Tobacco Smoking Withdrawal in Rats by Suppressing Neuroinflammation.Toxics. 2024 Aug 18;12(8):598. doi: 10.3390/toxics12080598. Toxics. 2024. PMID: 39195700 Free PMC article.
-
Rhoifolin as a potential anxiolytic drug for the effects of nicotine withdrawal: beneficial effects on behavior, neuroinflammation, and oxidative stress.Metab Brain Dis. 2025 May 20;40(5):208. doi: 10.1007/s11011-025-01627-5. Metab Brain Dis. 2025. PMID: 40392377
References
-
- Caputi F. F., Carboni L., Rullo L., Alessandrini I., Balzani E., Melotti R. M., et al. (2021). An exploratory pilot study of changes in global DNA methylation in patients undergoing major breast surgery under opioid-based general anesthesia. Front. Pharmacol. 12, 733577. 10.3389/fphar.2021.733577 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

