Delimiting MOGAD as a disease entity using translational imaging
- PMID: 38333186
- PMCID: PMC10851159
- DOI: 10.3389/fneur.2023.1216477
Delimiting MOGAD as a disease entity using translational imaging
Erratum in
-
Erratum: Delimiting MOGAD as a disease entity using translational imaging.Front Neurol. 2024 Mar 7;15:1390688. doi: 10.3389/fneur.2024.1390688. eCollection 2024. Front Neurol. 2024. PMID: 38515453 Free PMC article.
Abstract
The first formal consensus diagnostic criteria for myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) were recently proposed. Yet, the distinction of MOGAD-defining characteristics from characteristics of its important differential diagnoses such as multiple sclerosis (MS) and aquaporin-4 antibody seropositive neuromyelitis optica spectrum disorder (NMOSD) is still obstructed. In preclinical research, MOG antibody-based animal models were used for decades to derive knowledge about MS. In clinical research, people with MOGAD have been combined into cohorts with other diagnoses. Thus, it remains unclear to which extent the generated knowledge is specifically applicable to MOGAD. Translational research can contribute to identifying MOGAD characteristic features by establishing imaging methods and outcome parameters on proven pathophysiological grounds. This article reviews suitable animal models for translational MOGAD research and the current state and prospect of translational imaging in MOGAD.
Keywords: EAE; animal models; imaging; myelin oligodendrocyte glycoprotein associated disease; translational research.
Copyright © 2023 Oertel, Hastermann and Paul.
Conflict of interest statement
FP reports research support from Bayer, Novartis, Biogen, Teva, Sanofi-Aventis/Genzyme, Alexion, Roche and Merck Serono and research support from the German Research Council, Werth Stiftung of the City of Cologne, German Ministry of Education and Research, Arthur Arnstein Stiftung Berlin, EU FP7 Framework Program, Guthy-Jackson Charitable Foundation, and NMSS. He also reports receiving consultation fees as an associate editor for Neurology, Neuroimmunology, and Neuroinflammation and as an academic editor for PLoS ONE and consultant fees for Sanofi Genzyme, Biogen, MedImmune, Shire, and Alexion. He also reports receiving speaker honoraria from Bayer, Novartis, Biogen, Teva, Sanofi-Aventis/Genzyme, Merck Serono, Alexion, Chugai, MedImmune, and Shire. He is an advisory board member for Novartis and MedImmune Scientific and holds stocks of Nocturne GmbH—all outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

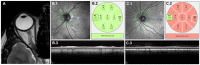
References
-
- Brunner C, Lassmann H, Waehneldt TV, Matthieu JM, Linington C. Differential ultrastructural localization of myelin basic protein, myelin/oligodendroglial glycoprotein, and 2′,3′-cyclic nucleotide 3′-phosphodiesterase in the CNS of adult rats. J Neurochem. (1989) 52:296–304. 10.1111/j.1471-4159.1989.tb10930.x - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

