Preoperative immune checkpoint inhibition and cryoablation in early-stage breast cancer
- PMID: 38333710
- PMCID: PMC10850740
- DOI: 10.1016/j.isci.2024.108880
Preoperative immune checkpoint inhibition and cryoablation in early-stage breast cancer
Abstract
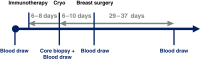
Local cryoablation can engender systemic immune activation/anticancer responses in tumors otherwise resistant to immune checkpoint blockade (ICB). We evaluated the safety/tolerability of preoperative cryoablation plus ipilimumab and nivolumab in 5 early-stage/resectable breast cancers. The primary endpoint was met when all 5 patients underwent standard-of-care primary breast surgery undelayedly. Three patients developed transient hyperthyroidism; one developed grade 4 liver toxicity (resolved with supportive management). We compared this strategy with cryoablation and/or ipilimumab. Dual ICB plus cryoablation induced higher expression of T cell activation markers and serum Th1 cytokines and reduced immunosuppressive serum CD4+PD-1hi T cells, improving effector-to-suppressor T cell ratio. After dual ICB and before cryoablation, T cell receptor sequencing of 4 patients showed increased T cell clonality. In this small subset of patients, we provide preliminary evidence that preoperative cryoablation plus ipilimumab and nivolumab is feasible, inducing systemic adaptive immune activation potentially more robust than cryoablation with/without ipilimumab.
Keywords: Health sciences; Immunology; Oncology.
© 2024 The Authors.
Conflict of interest statement
E.C. reports consulting for Pfizer, and Novartis. D.B.P. has served on advisory boards for Bristol Myers Squibb, Merck, Syndax, Nektar Therapeutics, Puma, Nanostring, Genentech, Brooklyn Immunotherapeutics, Sanofi, Biotheranostics, NGMBio, and Eli Lilly, received research funding from Bristol Myers Squibb, Merck, Brooklyn ImmunoTherapeutics, and WindMIL, and received speaking honoraria from Genentech and Novartis. P.W. reports consulting for Leap Therapeutics and uncompensated professional services/activities for Sellas Life Science Group. M.J. has received honoraria from Bayer and GE Healthcare. G.P. has served on advisory boards for Merck, Tizona, and Trishula Pharmaceuticals; provided professional services/activities for Paige.AI Inc; and received research funding from, and intellectual property rights with, Takeda. Y.B. has consulted for Hologic; provided professional services/activities for Boston Scientific and Pfizer; and received research grants from the Bristol Myers Squibb Foundation and the Society of Improved Medical Diagnosis. S.B.S. has consulted for Varian and received research grants from AngioDynamics, GE Healthcare, Elesta, and Johnson & Johnson; has equity in Adgero Biopharmaceuticals Inc, Aspire Bariatrics, EndoWays, Impulse Dynamics, Innoblative Designs, Johnson & Johnson, Lantheus Medical Imaging PC, Motus GI Holdings Inc, Poseida Therapeutics Inc, and SureFire LLC; has provided professional services/activities to Advantagene Inc, Microbot Medical Ltd, Olympus (compensated) and XACT Robotics Ltd (uncompensated); and has equity, fiduciary role/position, and intellectual property rights in Aperture Medical Technology. L.N. reports: Agenus Inc, Celgene Cold Spring Harbor Laboratory, QLS Advisors LLC (professional services/activities); American Society of Clinical Oncology (ASCO), Breast Cancer Research Foundation, NewStem Ltd, Springer Nature Limited, Translational Breast Cancer Research Consortium, United States Department of Justice (professional services and activities, uncompensated); Martell Diagnostic Laboratories Inc. (equity); Codagenix Inc, Immix Biopharma Inc (equity; professional services/activities); Cure Breast Cancer Foundation (intellectual property rights; professional services/activities, uncompensated), Samus Therapeutics LLC (equity; fiduciary role/position; professional services/activities, uncompensated). T.M. is a paid consultant for Immunos Therapeutics and Pfizer, is a co-founder and equity holder in IMVAQ Therapeutics, receives research support from Bristol Myers Squibb, Surface Oncology, Kyn Therapeutics, Infinity Pharmaceuticals, Inc., Peregrine Pharmaceuticals, Inc., Adaptive Biotechnologies, Leap Therapeutics, Inc., and Aprea, and is an inventor on patent applications related to work on oncolytic viral therapy, alpha virus-based vaccines, neoantigen modeling, CD40, GITR, OX40, PD-1, and CTLA-4. H.L.M. has consulted for Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Genentech/Roche, Immunomedics, Merck, OBI Pharma, Pfizer, Puma, Spectrum Pharmaceuticals, Syndax Pharmaceuticals, Peregrine, Calithera, Daiichi-Sankyo, Seattle Genetics, AstraZeneca, and TapImmune, and has received research support from Bristol Myers Squibb, MedImmune/AstraZeneca, BTG Ltd., and Merck. All reported research funding is administered by the institution. All disclosed relationships are outside the scope of the submitted work. The remaining authors declare no competing interests.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

