Dupilumab Treatment in Pediatric Patients Aged 6-11 Years with Severe Atopic Dermatitis Whose Disease Is Not Adequately Controlled: A Review
- PMID: 38333897
- PMCID: PMC10849907
- DOI: 10.2147/DDDT.S426947
Dupilumab Treatment in Pediatric Patients Aged 6-11 Years with Severe Atopic Dermatitis Whose Disease Is Not Adequately Controlled: A Review
Abstract
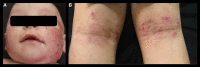
Atopic dermatitis (AD) is the most common inflammatory skin disease in children. Children with severe AD have a multidimensional disease burden characterized by skin lesions, itching, frequent infections, sleep deprivation, and a high rate of comorbidities. These impact the mental health and overall quality of life of not only the children but also of their parents and caregivers. There are few effective available treatment options for young children with severe AD that are suitable for long-term use. Due to their adverse effects, practice guidelines consider systemic agents inappropriate for this age group, although they are still used off-label in extreme cases. The biologic dupilumab has recently been approved for children aged 6-11 years with severe (EU) and moderate-to-severe (USA) AD, offering hope to this population of patients with a high unmet clinical need. The purpose of this review is to describe the unmet needs of AD patients aged 6-11 years prior to dupilumab approval and to summarize existing clinical data supporting dupilumab's safety and efficacy in these children.
Keywords: atopic dermatitis; children; dupilumab; pediatric; severe.
© 2024 Cork et al.
Conflict of interest statement
Professor Michael J Cork has served as a consultant and/or advisory board member and/or received research grants from Almirall, Amgen, Astellas Pharma, Bayer, GSK, Hyphens Pharma, Johnson & Johnson, LEO Pharma, L’Oréal, Novartis, Perrigo, Pfizer, Procter & Gamble, Sanofi, Stiefel, Regeneron Pharmaceuticals Inc., and Unilever. Professor Michael J Cork is a voluntary medical advisor to the National Eczema Society UK. Dr Simon G Danby reports grants from Sanofi, Almirall, Johnson & Johnson, and Leo Pharma; grants, personal fees from Perrigo, Pfizer, and Hyphens Pharma, outside the submitted work. Dr Ana B. Rossi is an employee of and may hold stock and/or stock options in Sanofi. Dr Ashish Bansal is an employee and shareholder of Regeneron Pharmaceuticals Inc.
Figures




References
-
- Paller AS, Guttman-Yassky E, Schuttelaar ML, et al. Disease characteristics, comorbidities, treatment patterns and quality of life impact in children <12 years old with atopic dermatitis: interim results from the PEDISTAD real-world registry. J Am Acad Dermatol. 2022;87(5):1104–1108. doi: 10.1016/j.jaad.2022.01.018 - DOI - PubMed
-
- Silverberg JI, Simpson EL, Weidinger S, et al. Children with atopic dermatitis (AD) have a high burden of atopic comorbidities: results from a large worldwide survey. J Allergy Clin Immunol. 2021;147(2):AB31. doi: 10.1016/j.jaci.2020.12.149 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

