Human Placenta-Derived Mesenchymal Stem Cells Combined With Artificial Dermal Scaffold Enhance Wound Healing in a Tendon-Exposed Wound of a Rabbit Model
- PMID: 38334047
- PMCID: PMC10858670
- DOI: 10.1177/09636897241228922
Human Placenta-Derived Mesenchymal Stem Cells Combined With Artificial Dermal Scaffold Enhance Wound Healing in a Tendon-Exposed Wound of a Rabbit Model
Abstract
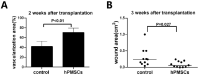
To overcome the difficulty of vascular regeneration in exposed tendon wounds, we combined human placenta-derived mesenchymal stem cells (hPMSCs) with an artificial dermal scaffold and assessed their role in promoting vascular regeneration and wound healing in vivo. hPMSCs were isolated from the human placenta and characterized based on their morphology, phenotypic profiles, and pluripotency. New Zealand rabbits were used to establish an exposed tendon wound model, and hPMSCs and artificial dermal scaffolds were transplanted into the wounds. The results of gross wound observations and pathological sections showed that hPMSCs combined with artificial dermal scaffold transplantation increased the vascularization area of the wound, promoted wound healing, and increased the survival rate of autologous skin transplantation. Following artificial dermal scaffold transplantation, hPMSCs accelerated the vascularization of the dermal scaffold, and the number of fibroblasts, collagen fibers, and neovascularization in the dermal scaffold after 1 week were much higher than those in the control group. Immunohistochemical staining further confirmed that the expression of the vascular endothelial cell marker, CD31, was significantly higher in the combined transplantation group than in the dermal scaffold transplantation group. Our findings demonstrated that hPMSCs seeded onto artificial dermal scaffold could facilitate vascularization of the dermal scaffold and improve tendon-exposed wound healing.
Keywords: angiogenesis; artificial dermal scaffold; placenta-derived mesenchymal stem cells; tendon exposure wound.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures







References
MeSH terms
LinkOut - more resources
Full Text Sources

