Unraveling the complexity of the senescence-associated secretory phenotype in adamantinomatous craniopharyngioma using multimodal machine learning analysis
- PMID: 38334125
- PMCID: PMC11145462
- DOI: 10.1093/neuonc/noae015
Unraveling the complexity of the senescence-associated secretory phenotype in adamantinomatous craniopharyngioma using multimodal machine learning analysis
Abstract
Background: Cellular senescence can have positive and negative effects on the body, including aiding in damage repair and facilitating tumor growth. Adamantinomatous craniopharyngioma (ACP), the most common pediatric sellar/suprasellar brain tumor, poses significant treatment challenges. Recent studies suggest that senescent cells in ACP tumors may contribute to tumor growth and invasion by releasing a senesecence-associated secretory phenotype. However, a detailed analysis of these characteristics has yet to be completed.
Methods: We analyzed primary tissue samples from ACP patients using single-cell, single-nuclei, and spatial RNA sequencing. We performed various analyses, including gene expression clustering, inferred senescence cells from gene expression, and conducted cytokine signaling inference. We utilized LASSO to select essential gene expression pathways associated with senescence. Finally, we validated our findings through immunostaining.
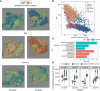
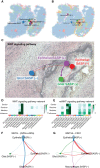
Results: We observed significant diversity in gene expression and tissue structure. Key factors such as NFKB, RELA, and SP1 are essential in regulating gene expression, while senescence markers are present throughout the tissue. SPP1 is the most significant cytokine signaling network among ACP cells, while the Wnt signaling pathway predominantly occurs between epithelial and glial cells. Our research has identified links between senescence-associated features and pathways, such as PI3K/Akt/mTOR, MYC, FZD, and Hedgehog, with increased P53 expression associated with senescence in these cells.
Conclusions: A complex interplay between cellular senescence, cytokine signaling, and gene expression pathways underlies ACP development. Further research is crucial to understand how these elements interact to create novel therapeutic approaches for patients with ACP.
Keywords: adamantinomatous craniopharyngioma (ACP); machine learning; next generation sequencing; pediatric neuro-oncology; senescence-associated secretory phenotype (SASP).
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
None declared.
Figures





References
-
- Wang L, Lankhorst L, Bernards R.. Exploiting senescence for the treatment of cancer. Nat Rev Cancer. 2022;22(6):340–355. - PubMed
-
- Kumari R, Jat P.. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol. 2021;9(1):485. https://www.frontiersin.org/articles/10.3389/fcell.2021.645593. Accessed April 25, 2023. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

