The Recent Progresses of Electrodes and Electrolysers for Seawater Electrolysis
- PMID: 38334510
- PMCID: PMC10856650
- DOI: 10.3390/nano14030239
The Recent Progresses of Electrodes and Electrolysers for Seawater Electrolysis
Abstract
The utilization of renewable energy for hydrogen production presents a promising pathway towards achieving carbon neutrality in energy consumption. Water electrolysis, utilizing pure water, has proven to be a robust technology for clean hydrogen production. Recently, seawater electrolysis has emerged as an attractive alternative due to the limitations of deep-sea regions imposed by the transmission capacity of long-distance undersea cables. However, seawater electrolysis faces several challenges, including the slow kinetics of the oxygen evolution reaction (OER), the competing chlorine evolution reaction (CER) processes, electrode degradation caused by chloride ions, and the formation of precipitates on the cathode. The electrode and catalyst materials are corroded by the Cl- under long-term operations. Numerous efforts have been made to address these issues arising from impurities in the seawater. This review focuses on recent progress in developing high-performance electrodes and electrolyser designs for efficient seawater electrolysis. Its aim is to provide a systematic and insightful introduction and discussion on seawater electrolysers and electrodes with the hope of promoting the utilization of offshore renewable energy sources through seawater electrolysis.
Keywords: chlorine evolution reaction; electrode; electrolyser; oxygen evolution reaction; seawater electrolysis.
Conflict of interest statement
Author Fan Zhang, Xiaofeng Chen and Shengxiao Zhao were employed by the company Power China Huadong Engineering Corporation Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
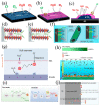




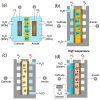
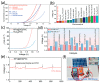





References
-
- Msheik M., Rodat S., Abanades S. Methane Cracking for Hydrogen Production: A Review of Catalytic and Molten Media Pyrolysis. Energies. 2021;14:3107. doi: 10.3390/en14113107. - DOI
-
- Wang C., Wang Y., Chen M., Liang D., Yang Z., Cheng W., Tang Z., Wang J., Zhang H. Recent advances during CH4 dry reforming for syngas production: A mini review. Int. J. Hydrogen Energy. 2021;46:5852–5874. doi: 10.1016/j.ijhydene.2020.10.240. - DOI
-
- Ulejczyk B., Nogal Ł., Młotek M., Krawczyk K. Enhanced production of hydrogen from methanol using spark discharge generated in a small portable reactor. Energy Rep. 2022;8:183–191. doi: 10.1016/j.egyr.2021.11.290. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

