Facile Synthesis of Ni-MgO/CNT Nanocomposite for Hydrogen Evolution Reaction
- PMID: 38334551
- PMCID: PMC10857693
- DOI: 10.3390/nano14030280
Facile Synthesis of Ni-MgO/CNT Nanocomposite for Hydrogen Evolution Reaction
Abstract
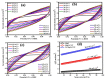
In this study, the pristine MgO, MgO/CNT and Ni-MgO/CNT nanocomposites were processed using the impregnation and chemical vapor deposition methods and analyzed for hydrogen evolution reaction (HER) using the electrochemical water splitting process. Furthermore, the effect of nickel on the deposited carbon was systematically elaborated in this study. The highly conductive carbon nanotubes (CNTs) deposited on the metal surface of the Ni-MgO nanocomposite heterostructure provides a robust stability and superior electrocatalytic activity. The optimized Ni-MgO/CNT nanocomposite exhibited hierarchical, helical-shaped carbon nanotubes adorned on the surface of the Ni-MgO flakes, forming a hybrid metal-carbon network structure. The catalytic HER was carried out in a 1M alkaline KOH electrolyte, and the optimized Ni-MgO/CNT nanocomposite achieved a low (117 mV) overpotential value (ɳ) at 10 mA cm-2 and needed a low (116 mV/dec) Tafel value, denotes the Volmer-Heyrovsky pathway. Also, the high electrochemical active surface area (ECSA) value of the Ni-MgO/CNT nanocomposite attained 515 cm2, which is favorable for the generation of abundant electroactive species, and the prepared electrocatalyst durability was also performed using a chronoamperometry test for the prolonged duration of 20 h at 10 mA cm-2 and exhibited good stability, with a 72% retention. Hence, the obtained results demonstrate that the optimized Ni-MgO/CNT nanocomposite is a highly active and cost-effective electrocatalyst for hydrogen energy production.
Keywords: chemical vapor deposition; hydrogen evolution reaction; hydrogen production; overpotential; water splitting.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Oxygen Plasma Activation of Carbon Nanotubes-Interconnected Prussian Blue Analogue for Oxygen Evolution Reaction.ACS Appl Mater Interfaces. 2020 Sep 23;12(38):42634-42643. doi: 10.1021/acsami.0c07821. Epub 2020 Sep 4. ACS Appl Mater Interfaces. 2020. PMID: 32845608
-
Polyolefin-derived substrate-grown carbon nanotubes as binder-free electrode for hydrogen evolution in alkaline media.Chemosphere. 2024 Feb;349:140769. doi: 10.1016/j.chemosphere.2023.140769. Epub 2023 Nov 22. Chemosphere. 2024. PMID: 38000550
-
Highly Electroactive Ni Pyrophosphate/Pt Catalyst toward Hydrogen Evolution Reaction.ACS Appl Mater Interfaces. 2019 Feb 6;11(5):4969-4982. doi: 10.1021/acsami.8b18153. Epub 2019 Jan 23. ACS Appl Mater Interfaces. 2019. PMID: 30624046
-
Synthesis of Ketjenblack Decorated Pillared Ni(Fe) Metal-Organic Frameworks as Precursor Electrocatalysts for Enhancing the Oxygen Evolution Reaction.Molecules. 2023 May 31;28(11):4464. doi: 10.3390/molecules28114464. Molecules. 2023. PMID: 37298940 Free PMC article.
-
Metal-Organic Polymer-Derived Interconnected Fe-Ni Alloy by Carbon Nanotubes as an Advanced Design of Urea Oxidation Catalysts.ACS Appl Mater Interfaces. 2021 Feb 24;13(7):8461-8473. doi: 10.1021/acsami.0c22148. Epub 2021 Feb 10. ACS Appl Mater Interfaces. 2021. PMID: 33565313
Cited by
-
Efficient Zinc Vanadate Homojunction with Cadmium Nanostructures for Photocatalytic Water Splitting and Hydrogen Evolution.Nanomaterials (Basel). 2024 Mar 9;14(6):492. doi: 10.3390/nano14060492. Nanomaterials (Basel). 2024. PMID: 38535640 Free PMC article.
References
-
- Wang M., Wang Z., Gong X., Guo Z. The intensification technologies to water electrolysis for hydrogen production—A review. Renew. Sustain. Energy Rev. 2014;29:573–588. doi: 10.1016/j.rser.2013.08.090. - DOI
-
- Susmozas A., Iribarren D., Dufour J. Life-cycle performance of indirect biomass gasification as a green alternative to steam methane reforming for hydrogen production. Int. J. Hydrogen Energy. 2013;38:9961–9972. doi: 10.1016/j.ijhydene.2013.06.012. - DOI
-
- Li X., Hao X., Abudula A., Guan G. Nanostructured catalysts for electrochemical water splitting: Current state and prospects. J. Mater. Chem. A. 2016;4:11973–12000. doi: 10.1039/C6TA02334G. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

